Use of Outpatient Pharmacological Treatment Among Infants Born with Neonatal Abstinence Syndrome: Medicaid 2008-2017
ASPE ISSUE BRIEF
Mir M. Ali, Emma Nye, and Kristina West
U.S. Department of Health and Human Services
Office of the Assistant Secretary for Planning and Evaluation
December 2020
Link to Printer Friendly Version in PDF Format (5 PDF pages)
ABSTRACT: This paper examines trends in outpatient pharmacological treatment among infants with Neonatal Abstinence Syndrome (NAS) between 2008 and 2017 in the United States, using a multi-state Medicaid claims database.
This brief was prepared through intramural research by the U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation, Office of Behavioral Health, Disability, and Aging Policy. For additional information about this subject, you can visit the BHDAP home page at https://aspe.hhs.gov/bhdap or contact the authors at HHS/ASPE/BHDAP, Room 424E, H.H. Humphrey Building, 200 Independence Avenue, S.W., Washington, D.C. 20201, Mir.Ali@hhs.gov, Emma.Nye@hhs.gov, Kristina.West@hhs.gov.
DISCLAIMER: The opinions and views expressed in this brief are those of the authors. They do not reflect the views of the Department of Health and Human Services, the contractor or any other funding organization. This brief was completed and submitted in March 2020.
| HIGHLIGHTS This paper examines trends in outpatient pharmacological treatment among infants with NAS between 2008 and 2017 in the United States, using a multi-state Medicaid claims database. Key findings include the following:
|
Introduction
The rate of Neonatal Abstinence Syndrome (NAS) increased rapidly in the United States during the last decade as the opioid crisis expanded across the country (Wahlen et al., 2019). Recent policy efforts around NAS have focused on providing evidence-based treatment for both the infants and their mothers, increasing the accessibility of family-friendly services for pregnant and parenting women with opioid use disorder and supporting continuing education for health care providers. For example, the 2015 Protecting Our Infants Act (P.L. 114-91) sought to address health problems related to prenatal opioid exposure and NAS, and required the U.S. Department of Health and Human Services to study and develop recommendations for the prevention, identification, and treatment of NAS. In addition, the Comprehensive Addiction and Recovery Act of 2016 (P.L. 114-198) includes a provision to examine NAS in the United States and related treatment services for the condition.
NAS infants tend to experience longer hospital stays, higher rates of readmissions, and higher treatment costs compared to non-NAS infants (Winkelman et al., 2018). Although the preferred first-line treatment varies across hospitals in the United States, existing guidelines suggest non-pharmacologic treatments for infants with mild symptoms, and pharmacological intervention, in addition to non-pharmacological treatments, for infants with moderate to severe signs of NAS (SAMHSA, 2018). The recommended medications for infants with NAS symptoms are liquid oral morphine and liquid oral methadone. Oral liquid clonidine, or oral liquid phenobarbital can be used as adjuvants for infants with severe NAS not adequately relieved by morphine or methadone (SAMHSA, 2018). Recent research suggests that buprenorphine has the most favorable outcomes for infants, but more research is needed before buprenorphine can be recommended for infants with NAS (Disher et al., 2019).
It is estimated that between 50% and 80% of the pharmacological treatment of NAS takes place in an inpatient setting, particularly within newborn intensive care units (Wachman and Werler, 2019). There is evidence that some providers continue an outpatient weaning regimen, despite the lack of consensus on standards of care to guide outpatient weaning at present (Wachman and Werler, 2019; Disher et al., 2019). A limited but growing literature has suggested that a combination of inpatient and outpatient pharmacologic treatment may be associated with a shorter average length of hospital stay and reduced treatment cost for NAS infants (Lee et al., 2015). However, not much is known about the use of outpatient pharmacological treatment among NAS infants. Using Medicaid claims data, this report aims to estimate trends in outpatient pharmacological treatment in NAS infants between 2008 and 2017 in the United States.
Among Infants Born with NAS, the Percentage Receiving Outpatient Pharmacological Treatment Began Low and Declined
Approximately one out of seven infants diagnosed with NAS received any outpatient pharmacological treatment throughout the study period (Figure 1). Fifteen percent of NAS infants received any outpatient pharmacological treatment in 2008 and the rates declined through the end of the study period. The rate of outpatient pharmacological treatment was between 15% and 13% during 2008-2012 and then experienced a decline to reach 8% in 2014 and eventually 5% in 2017.
| FIGURE 1. Outpatient Pharmacological Treatment among Medicaid NAS Infants within 3 Months of Birth |
|---|
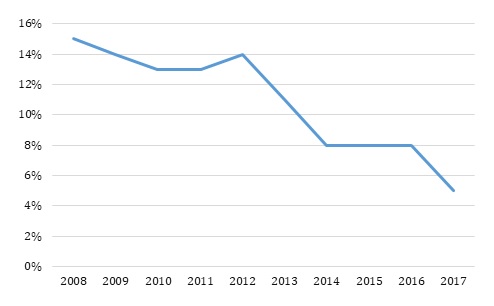 |
The study used IBM Watson Health MarketScan Multi-State Medicaid claims database (2008-2017). The sample includes all infants (ICD-9 livebirth codes V30-V39 and ICD-10 livebirth codes Z37-Z38) diagnosed with NAS (ICD-9 code 779.5 and ICD-10 code P961) born between 2008 and 2017. The sample size ranged from 989 NAS infants in 2008 to 5,027 NAS infants in 2017. Outpatient pharmacological treatment among NAS infants was measured by whether the infants had any morphine, methadone, buprenorphine, phenobarbital, or clonidine prescription fills within 90 days of release from the hospital for each of the study years.
Discussion
Outpatient pharmacological treatment among NAS infants covered under Medicaid remained low and declined between 2008 and 2017. Pharmacological treatment has been shown to be effective in situations where infants are not responsive to non-pharmacological care (Wahlen et al., 2019; SAMHSA, 2018). Therefore, increased policy focus to develop guidelines for identifying when pharmacological intervention is needed and to support implementation to ensure the necessary treatment is administered successfully might be helpful (CMCS, 2019; SAMHSA, 2017).
The findings of this study should be viewed in the context of some limitations. First, the low rates of outpatient pharmacological treatment might be due to the completion of such treatment during an infant's hospital inpatient stay immediately following birth. MarketScan Medicaid data only captures outpatient pharmacy claims and does not allow the identification of prescriptions administered during hospital stays, so this supposition cannot be confirmed. Second, the number of Medicaid states represented in the MarketScan data varies from year to year; trends in the pharmacological treatment rate observed in this study could be a function of this inconsistency. Finally, given the absence of a standard of pharmacological treatment protocol for NAS infants, the low and declining rates of such treatment may indicate greater use of non-pharmacological treatment. Most non-pharmacological care options are not billable treatments and thus cannot be measured using claims database.
That pharmacological treatment rates are declining is surprising given this is occurring concomitantly with increasing public awareness of the effects of the opioid epidemic and its impact on children and families. More research is needed to understand whether the declining pharmacological treatment for NAS is associated with increased in-hospital pharmacological treatment or non-pharmacological approaches, or if the decline is due to other factors including provider attitudes.
References
Center for Medicaid and CHIP Services. (2019). State Guidance for Implementation of the Treatment for Infants with Neonatal Abstinence Syndrome in Residential Pediatric Recovery Centers provisions of Section 1007 of Pub. L. 115-271, the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act. CMCS Informational Bulletin. Accessed September 8, 2019, at https://www.medicaid.gov/federal-policy-guidance/downloads/cib072619-1007.pdf.
Disher T, Gullickson C, Singh B, Cameron C, Boulos L, Beaubien L, Campbell-Yeo M. (2019). Pharmacological treatments for neonatal abstinence syndrome: a systematic review and network meta-analysis. JAMA Pediatrics; 173(3): 234-243.
Lee J, Hulman S, Musci M Jr, Stang E. (2015). Neonatal Abstinence Syndrome: Influence of a combined inpatient/outpatient methadone treatment regimen on the average length of stay of a Medicaid NICU population. Population Health Management; 18(5): 392-397.
Substance Abuse and Mental Health Services Administration (SAMHSA). (2018). Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Accessed April 10, 2019, at https://store.samhsa.gov/product/Clinical-Guidance-for-Treating-Pregnant-and-Parenting-Women-With-Opioid-Use-Disorder-and-Their-Infants/SMA18-5054.
Substance Abuse and Mental Health Services Administration (SAMHSA). (2017). State Pilot Grant Program for Treatment for Pregnant and Postpartum Women. SAMHSA Funding Opportunity Announcement. Accessed September 8, 2019, at https://www.samhsa.gov/grants/grant-announcements/ti-17-016.
Wachman EM, Werler MM. (2019). Pharmacologic Treatment for Neonatal Abstinence Syndrome: Which medication is Best? JAMA Pediatrics; 173(3): 221-223.
Wahlen BL, Holmes AV, Blythe S. (2019). Models of care for neonatal abstinence syndrome: What works? Seminars in Fetal & Neonatal Medicine; 24(2): 121-132.
Winkelman TA, Villapiano N, Kozhimannil KB, Davis MM, Patrick S. (2018). Incidence and costs of Neonatal Abstinence Syndrome among infants with Medicaid: 2004-2014. Pediatrics; 141(4): e20173520.
Neonatal Abstinence Syndrome
This brief was prepared through intramural research by the U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation, Office of Behavioral Health, Disability, and Aging Policy. For additional information about this subject, you can visit the BHDAP home page at https://aspe.hhs.gov/bhdap or contact the authors at HHS/ASPE/BHDAP, Room 424E, H.H. Humphrey Building, 200 Independence Avenue, S.W., Washington, D.C. 20201, Mir.Ali@hhs.gov, Emma.Nye@hhs.gov, Kristina.West@hhs.gov.
Reports Available
Trends in Hospital Readmission and Emergency Department Visit among Infants Born with Neonatal Abstinence Syndrome Issue Brief
- HTML version: https://aspe.hhs.gov/basic-report/trends-hospital-readmission-and-emergency-department-visit-among-infants-born-neonatal-abstinence-syndrome-issue-brief
- PDF version: https://aspe.hhs.gov/pdf-report/trends-hospital-readmission-and-emergency-department-visit-among-infants-born-neonatal-abstinence-syndrome-issue-brief
Use of Outpatient Pharmacological Treatment Among Infants Born with Neonatal Abstinence Syndrome: Medicaid 2008-2017 Issue Brief
- HTML version: https://aspe.hhs.gov/basic-report/use-outpatient-pharmacological-treatment-among-infants-born-neonatal-abstinence-syndrome-medicaid-2008-2017-issue-brief
- PDF version: https://aspe.hhs.gov/pdf-report/use-outpatient-pharmacological-treatment-among-infants-born-neonatal-abstinence-syndrome-medicaid-2008-2017-issue-brief
Utilization of Mental Health Services among Children Diagnosed with Neonatal Abstinence Syndrome at Birth Issue Brief
- HTML version: https://aspe.hhs.gov/basic-report/utilization-mental-health-services-among-children-diagnosed-neonatal-abstinence-syndrome-birth-issue-brief
- PDF version: https://aspe.hhs.gov/pdf-report/utilization-mental-health-services-among-children-diagnosed-neonatal-abstinence-syndrome-birth-issue-brief
