- National Alzheimer’s Project Act
- Alzheimer’s Disease and Related Dementias
- The Challenges
- Framework and Guiding Principles
- Goals as Building Blocks for Transformation
- 2021 Update
Goal 1: Prevent and Effectively Treat Alzheimer’s Disease and Related Dementias by 2025
- Strategy 1.A: Identify Research Priorities and Milestones
- Strategy 1.B: Expand Research Aimed at Preventing and Treating Alzheimer’s Disease and Related Dementias
- Strategy 1.C: Accelerate Efforts to Identify Early and Presymptomatic Stages of Alzheimer’s Disease and Related Dementias
- Strategy 1.D: Coordinate Research with International Public and Private Entities
- Strategy 1.E: Facilitate Translation of Findings into Medical Practice and Public Health Programs
Goal 2: Enhance Care Quality and Efficiency
- Strategy 2.A: Build a Workforce with the Skills to Provide High-Quality Care
- Strategy 2.B: Ensure Timely and Accurate Diagnosis
- Strategy 2.C: Educate and Support People with Alzheimer’s Disease and Related Dementias and Their Families upon Diagnosis
- Strategy 2.D: Identify High-Quality Dementia Care Guidelines and Measures Across Care Settings
- Strategy 2.E: Explore the Effectiveness of New Models of Care for People with Alzheimer’s Disease and Related Dementias
- Strategy 2.F: Ensure that People with Alzheimer’s Disease and Related Dementias Experience Safe and Effective Transitions between Care Settings and Systems
- Strategy 2.G: Advance Coordinated and Integrated Health and Long-Term Services and Supports for People Living with Alzheimer’s Disease and Related Dementias
- Strategy 2.H: Improve Care for Populations Disproportionally Affected by Alzheimer’s Disease and Related Dementias, and for Populations Facing Care Challenges
Goal 3: Expand Supports for People with Alzheimer’s Disease and Related Dementias and Their Families
- Strategy 3.A: Ensure Receipt of Culturally and Linguistically Appropriate Education, Training, and Support Materials
- Strategy 3.B: Enable Family Caregivers to Continue to Provide Care while Maintaining Their Own Health and Well-Being
- Strategy 3.C: Assist Families in Planning for Future Care Needs
- Strategy 3.D: Maintain the Dignity, Safety and Rights of People with Alzheimer’s Disease and Related Dementias
- Strategy 3.E: Assess and Address the Long-Term Services and Supports Needs of People with Alzheimer’s Disease and Related Dementias
Goal 4: Enhance Public Awareness and Engagement
- Strategy 4.A: Educate the Public about Alzheimer’s Disease and Related Dementias
- Strategy 4.B: Work with State, Tribal, and Local Governments to Improve Coordination and Identify Model Initiatives to Advance Alzheimer’s Disease and Related Dementias Awareness and Readiness across the Government
- Strategy 4.C: Coordinate United States Efforts with Those of the Global Community
Goal 5: Improve Data to Track Progress
- Strategy 5.A: Enhance the Federal Government’s Ability to Track Progress
- Strategy 5.B: Monitor Progress on the National Plan
Goal 6: Accelerate Action to Promote Healthy Aging and Reduce Risk Factors for Alzheimer’s Disease and Related Dementias
- Strategy 6.A: Identify Research Priorities and Expand Research on Risk Factors for Alzheimer’s Disease and Related Dementias
- Strategy 6.B: Facilitate Translation of Risk Reduction Research Findings into Clinical Practice
- Strategy 6.C: Accelerate Public Health Action to Address the Risk Factors for Alzheimer’s Disease and Related Dementias
- Strategy 6.D: Expand Interventions to Reduce Risk Factors, Manage Chronic Conditions, and Improve Well-Being through the Aging Network
- Strategy 6.E: Address Inequities in Risk Factors for Alzheimer’s Disease and Related Dementias Among Marginalized Populations
- Strategy 6.F: Engage the Public about Ways to Reduce Risks for Alzheimer’s Disease and Related Dementias
Appendix 1: List of Participating Departments and Agencies
Introduction
National Alzheimer’s Project Act
On January 4, 2011, the National Alzheimer's Project Act (NAPA) (Public Law 111-375) was signed into law. The Act defines "Alzheimer's" as Alzheimer's disease and Alzheimer’s disease-related dementias (AD/ADRD) and requires the Secretary of the U.S. Department of Health and Human Services (HHS) to establish the National Alzheimer's Project to:
- Create and maintain an integrated National Plan to overcome Alzheimer's disease;
- Coordinate Alzheimer's disease research and services across all federal agencies;
- Accelerate the development of treatments that would prevent, halt, or reverse the course of Alzheimer's disease;
- Improve early diagnosis and coordination of care and treatment of Alzheimer's disease;
- Decrease disparities in Alzheimer's disease for racial and ethnic minority populations that are at higher risk for Alzheimer's disease; and,
- Coordinate with international bodies to fight Alzheimer's disease globally.
The law also establishes the Advisory Council on Alzheimer's Research, Care, and Services (Advisory Council) and requires the Secretary of HHS, in collaboration with the Advisory Council, to create and maintain a National Plan to overcome AD/ADRD.
NAPA offers a historic opportunity to address the many challenges facing people with AD/ADRD and their families. Given the great demographic shifts that will occur over the next 30 years, including the doubling of the population of older adults, the success of this effort is of great importance to people with AD/ADRD and their family members, caregivers, public policy makers, and health and social service providers.
Alzheimer’s Disease and Related Dementias
Alzheimer's disease (AD) is an irreversible, progressive brain disease that affects as many as 5.5 million Americans.[1] It slowly destroys brain function, leading to cognitive decline (e.g., memory loss, language difficulty, poor executive function), behavioral and psychiatric disorders (e.g., depression, delusions, agitation), and declines in functional status (e.g., ability to engage in activities of daily living (ADLs) and self-care).[2] In 1906, Dr. Alois Alzheimer first documented the disease when he identified changes in the brain tissue of a woman who had memory loss, language problems, and unpredictable behavior. Her brain tissue included abnormal clumps (amyloid plaques) and tangled bundles of fibers (neurofibrillary tangles). Brain plaques and tangles, in addition to the loss of connections between neurons, are the main pathological features of AD.[3] However, other pathologic features occur commonly in the brain of older Americans diagnosed with AD, and these are thought to also contribute to the burden of dementia in the United States.[3, 4]
In addition to AD, this National Plan addresses Alzheimer's disease-related dementias (ADRD) consistent with the approach Congress used in NAPA. ADRD include frontotemporal dementia (FTD), Lewy body dementia (LBD), vascular contributions to cognitive impairment and dementia (VCID), and mixed dementias -- especially AD mixed with cerebrovascular disease or Lewy bodies. It is often difficult to distinguish between AD and ADRD in terms of clinical presentation and diagnosis. Some of the basic neurodegenerative processes have common pathways. Many people have the pathology of more than one type of dementia in their brains.[5] People with all forms of dementia and their families and caregivers face similar challenges in finding appropriate and necessary medical care and community-based services. As such, many of the actions described in this plan are designed to address these conditions collectively.
The first symptom of AD/ADRD is often memory impairment; however, poor attention and executive function, behavioral disorders, visual disturbances, sleep disruption or motor symptoms can often be the presenting symptoms. As the disease progresses, memory can decline, and other functions like language skills and decision making become more difficult. Personality and behavior changes often occur. Over time, a person with the disease may no longer recognize family and friends. Eventually, many persons who survive with AD/ADRD are completely reliant on others for assistance with even the most basic ADLs, such as eating, dressing, and bathing.[4, 6]
In more than 90% of people with AD/ADRD, symptoms do not appear until after age 60, and the incidence of the disease increases with age from 5.3% among adults ages 65-74 to 34.6% among adults aged 85 and older.[7] The causes of AD/ADRD are not completely understood, but researchers believe they include a combination of genetic, environmental, and lifestyle factors.[4] The importance of any one of these factors in increasing or decreasing the risk of developing AD/ADRD may differ from person to person. In rare cases, known as early-onset or younger-onset dementia, people develop symptoms in their 30s, 40s, or 50s. A significant number of people with Down syndrome develop dementia in their 50s or younger, often placing increased burden on their families and caregivers. The relative risk of dementia is higher in rural than urban areas, particularly among minority populations. Nationally, Black Americans are twice as likely and Hispanic or Latino (Hispanic) Americans are 1.5-times as likely to develop AD/ADRD compared to White Americans.[8, 9]
AD/ADRD is a major public health issue and will increasingly affect the health and well-being of the population. Unless the diseases can be effectively treated or prevented, the number of Americans with AD/ADRD will increase significantly in the next 2 decades as the population ages. The Bureau of the Census estimates that the number of people age 65 and older in the United States will almost double, to 88 million by 2050. The prevalence of people with AD/ADRD doubles for every 5-year interval beyond age 65. Without a preventive treatment or cure, the significant growth in the population over age 85 that is estimated to occur between 2015 and 2050 (from 6.3 million to 19 million) suggests a substantial increase in the number of people with AD/ADRD.
Significant emotional, physical, and financial stress is placed on individuals with AD/ADRD and their family members. Unpaid caregivers, often family members and friends, provide the majority of care for people with AD/ADRD in the community. Unpaid caregivers frequently do not identify themselves as such; they may be a wife, daughter, husband, parent, son, or friend helping a person whom they care about. However, the intensive support required for a person with AD/ADRD can negatively impact the caregiver's emotional and physical health and well-being and their ability to work. Unpaid caregivers often report symptoms of depression and anxiety, and they have poorer health outcomes than their peers who do not provide such care.[6]
Dementia care costs are significant and often a burden to families and others providing unpaid care. Recent estimates from one nationally representative study found that paid and unpaid care costs for people older than age 70 with dementia in the United States in 2010 were between $159 billion and $215 billion. These figures include direct medical expenditures, costs for long-term services and supports (LTSS) including institutional and home and community-based services (HCBS), and two different estimates of the value of unpaid care provided by family members and friends. These costs could rise dramatically with the increase in the numbers of older adults in coming decades. Care costs per person with dementia in 2010 ranged from $75,000 to $83,000 depending on how unpaid care costs were estimated.[10] These national dementia care costs are comparable to, if not greater than, those for heart disease and cancer.[11]
Caring for people with the disease also strains health and long-term care systems. Individuals with AD/ADRD use a disproportionate amount of health care resources; for instance, they are hospitalized 2-3 times as often as people of the same age who do not have the disease.[12] Similarly, estimates from national data show that nearly seven out of ten residents in assisted living residences have some form of cognitive impairment.[13] As the number of people with AD/ADRD grows over the next 3 decades, these diseases will place a major strain on these care systems as well as on Medicare and Medicaid, the major funders of institutional, clinical care, and HCBS. Although Medicaid, a program for eligible low income Americans, covers long-term care such as nursing home care and HCBS, Medicare does not. Most Americans underestimate the risk of disability and the need for long-term care. More than half of older adults turning 65 today will develop a disability such as AD/ADRD serious enough to require LTSS, although most will need assistance for less than 2 years. About one in seven will have a disability for more than 5 years. On average, an American turning 65 today will incur $138,000 in future LTSS costs. Families will pay about half of the costs themselves out-of-pocket with the rest covered by current public programs and private insurance.[14]
The Challenges
The National Plan was designed to address the major challenges presented by AD/ADRD:
-
While research on AD/ADRD has made steady progress, there are no pharmacological or other interventions known to definitively prevent, treat, or cure the diseases.
-
While HHS and other groups have taken steps to develop quality measures to assess dementia care and to improve the training of the health and long-term care workforce -- for both paid and unpaid caregivers -- there is room for improvement.
-
Family members and other unpaid caregivers, who take on the responsibility of caring for a person with AD/ADRD, also need services and supports. The majority of people with AD/ADRD live in the community, where their families provide most of their care. The toll of caregiving can have major implications for caregivers and families as well as population health, with about one-third of caregivers reporting symptoms of depression.[13, 15]
-
Stigmas and misconceptions associated with AD/ADRD are widespread and profoundly impact the care provided to and the isolation felt by people with AD/ADRD and their families and caregivers.
-
Public and private sector progress is significant but should be coordinated and tracked. In addition, data to track the incidence, prevalence, trajectory, and costs of AD/ADRD are limited.
Framework and Guiding Principles
The enactment of NAPA provided an opportunity to focus the Nation's attention on the challenges of AD/ADRD. In consultation with stakeholders both inside and outside of the Federal Government, this National Plan represents the blueprint for achieving the vision of a nation free of AD/ADRD.
Central to and guiding the National Plan are the people most intimately impacted by AD/ADRD -- those who have the diseases and their families and other caregivers. Individuals with AD/ADRD and their caregivers receive assistance from both the clinical health care system and long-term care including HCBS, legal services, and other social services. Both the clinical care and community/support environments need better tools to serve people with AD/ADRD and their unpaid caregivers. Ongoing and future research seeks to identify interventions to assist clinicians, supportive service providers, HCBS providers, persons living with dementia, and caregivers. All of these efforts must occur in the context of improved awareness of the diseases, their risk factors, and their impacts, as well as opportunities for improvement. The Plan aims to address these key needs. HHS is committed to tracking and coordinating the implementation of NAPA and making improvements aimed at achieving its ambitious vision.
The National Plan continues to be guided by three principles:
- Optimize Existing Resources and Improve and Coordinate Ongoing Activities. The first step in developing the National Plan was to set up a federal interagency working group and conduct an inventory of all federal activities involving AD/ADRD. In creating the Plan, HHS and its partners sought to leverage these resources and activities, improve coordination, and reduce duplication of efforts to better meet the challenges of AD/ADRD. The activities included in the inventory comprise ongoing work and new opportunities. The federal working group process continues to improve coordination and awareness throughout the Federal Government and set in motion commitments for further collaboration. Further, this process has allowed for identification of non-AD-specific programs and resources that may be leveraged to advance AD/ADRD care and prevention.
- Support Public-Private Partnerships. The scope of the challenges of AD/ADRD is so great that partnerships with a multitude of public and private stakeholders are essential to making progress. The original National Plan began the partnership process by identifying areas of need and opportunity. The Plan continues to rely on the Advisory Council in particular to identify key areas where public-private partnerships can improve outcomes.
- Transform the Way We Approach Alzheimer's Disease and Related Dementias. The National Plan recognizes that this undertaking will require continued, large-scale, coordinated efforts across the public and private sectors. With principles 1 and 2 above, as well as the ambitious vision that the Federal Government has committed to through this Plan, HHS and its federal partners continue to take transformative action needed to address these diseases. With ongoing input from the Advisory Council, the Federal Government continues to identify the most promising areas for progress and marshal resources from both within and outside the government to act on these opportunities.
Goals as Building Blocks for Transformation
Achieving the vision of eliminating the burden of AD/ADRD starts with concrete goals. Below are the five that form the foundation of the National Plan:
- Prevent and Effectively Treat Alzheimer's Disease and Related Dementias by 2025.
- Enhance Care Quality and Efficiency.
- Expand Supports for People with Alzheimer's Disease and Related Dementias and their Families.
- Enhance Public Awareness and Engagement.
- Track Progress and Drive Improvement.
2021 Update
This is the ninth Update to the National Plan. In addition to the five goals mentioned above, in 2021 a sixth goal is being added:
- Accelerate Action to Promote Healthy Aging and Reduce Risk Factors for Alzheimer’s Disease and Related Dementias
In recognition of the advances in understanding of risk factors for AD/ADRD, in July 2020 the Advisory Council on Alzheimer’s Research, Care, and Services recommended the creation of a subcommittee focused on modifiable risk factors. The subcommittee was charged with exploring the evidence and ways to reduce the burden of risk factors to prevent or delay onset of AD/ADRD. Subcommittee members represented various areas of expertise, including research, public health, innovation, and clinical care and were from diverse racial, ethnic, and geographical backgrounds. The subcommittee reviewed the evidence and received extensive stakeholder feedback. In July 2021, the subcommittee recommended that HHS add a sixth goal to the National Plan to address AD/ADRD focused on risk reduction. The subcommittee’s recommendation was adopted by the Advisory Council on July 19, 2021.
Based on this recommendation and the latest research, HHS and its federal partners added this sixth goal, Accelerate Action to Promote Healthy Aging and Reduce Risk Factors for Alzheimer’s Disease and Related Dementias to the National Plan in this 2021 Update. Under the new goal, the federal agencies will advance and expand research on risk factors for AD/ADRD. They will also strengthen the infrastructure needed to disseminate information about risk factors, interventions to address them, and related health promotion activities to health care and community providers and to public health networks.
Figure 1 shows how all six goals cover the issues related to AD/ADRD throughout the disease trajectory.
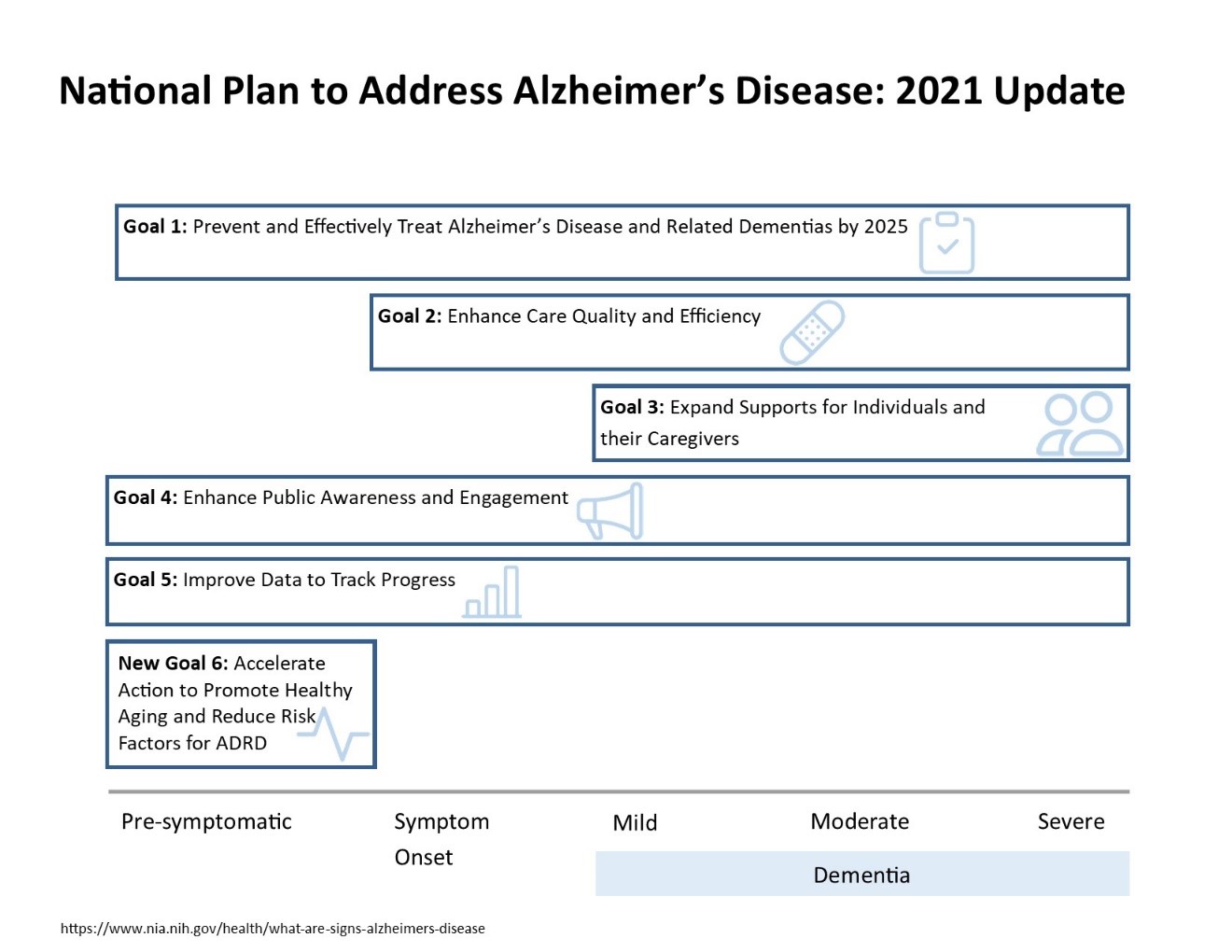
As of December 2021, the United States is still in the midst of the COVID-19 pandemic. The sheer volume of federal activities to address the pandemic, support older adults at highest risk of COVID-19 infection and mortality, and vaccinate Americans of all ages, is too great to include in this Update and goes well beyond the scope of the National Plan. Instead, agencies have had to adapt their programs, activities, and interventions to navigate the challenges of the pandemic as seamlessly and effectively as possible. Integrating their work into the existing framework of the National Plan is meant to represent those ongoing processes and achievements.
Goal 1: Prevent and Effectively Treat Alzheimer’s Disease and Related Dementias by 2025
Research continues to expand our understanding of the causes of, treatments for, and prevention of AD/ADRD. For example, in 2021 the Food and Drug Administration (FDA) approved aducanumab under the accelerated approval pathway. This goal seeks to develop additional prevention and treatment modalities by 2025. Ongoing research and clinical inquiry can inform our ability to prevent AD/ADRD, minimize its symptoms, and delay its progression. Under this goal, HHS will prioritize and accelerate the pace of scientific research and ensure that as evidence-based solutions are identified, they are quickly translated, put into practice, and brought to scale so that individuals with AD/ADRD can benefit from increases in scientific knowledge. HHS will identify interim milestones and set ambitious deadlines for achieving these milestones in order to meet this goal.
Strategy 1.A: Identify Research Priorities and Milestones
Research agencies undertake research planning processes on an ongoing basis, but a special effort is needed to identify the priorities and milestones to achieve Goal 1. The actions below will identify the priorities, establish milestones, and ensure that appropriate stakeholders are involved in the planning process aimed at preventing AD/ADRD and minimizing it as a health burden by 2025. During the course of this work, National Institutes of Health (NIH) and partner agencies will develop research priorities and a plan for implementing each phase of research in a coordinated manner.
(UPDATED) Action 1.A.1: Regularly convene an Alzheimer’s disease research summit to update priorities
Lead Agency: NIH/NIA
Partners: national and international experts, public and private stakeholders, academia, industry, professional and advocacy groups
The 2021 Alzheimer’s Disease Research Summit was held virtually in April. This was the fourth such summit, with previous summits occurring in 2012, 2015, and 2018. The summits bring together a multi-stakeholder community, including government, industry, academia, private foundation, and patient advocacy groups, to identify research priorities and further translate AD/ADRD research findings into practice. The goal is to accelerate the development of effective, disease-modifying, and palliative therapies for the cognitive as well as neuropsychiatric symptoms of Alzheimer's. The 2021 Summit built on the foundation laid through the work of the previous summit participants. Participants provided individual input that showcased progress to date and identified further gaps and opportunities toward the goal of precision medicine for AD/ADRD treatment and prevention. NIH is committed to regularly updating its research priorities and plans are underway for the next AD Research Summit in 2024.
For more information see:
(ONGOING) Action 1.A.2: Solicit diverse community input on Alzheimer’s disease research priorities
Lead Agency: NIA
National research summits (including the Alzheimer’s Disease Research Summit, Alzheimer’s Disease-Related Dementias Summit, and National Research Summit on Care, Services, and Supports for Persons with Dementia and their Caregivers) are held yearly on a rotating basis to gather scientific input and identify gaps and opportunities. This information factors into NIH’s research plan for the 2025 goal, which is outlined as a series of research implementation milestones. These milestones and the accompanying milestone database are updated annually based on this diverse input. This planning process and its systematic updates have informed the research community about NIH’s interests and priorities in funding projects in AD/ADRD. As of July 2020, the milestone database now includes better tracking of progress including success criteria and specific implementation activities.
For more information, see:
- https://aspe.hhs.gov/alzheimers-disease-related-dementias-adrd-summit-2016-prioritized-research-milestones
- https://www.nia.nih.gov/2020-dementia-care-summit
- https://www.nia.nih.gov/2021-alzheimers-summit
- https://www.nia.nih.gov/research/milestones
(ONGOING) Action 1.A.3: Regularly update the National Plan and refine Goal 1 strategies and action items based on feedback and input
Lead Agency: ASPE
Partners: NAPA Advisory Council, NIH/NIA
HHS and its federal partners will use the diverse input received through the Research Summits on AD/ADRD and on Care and Services to inform implementation of the National Plan. An updated Goal 1 will reflect the priorities, milestones, and timeline elements identified through these processes to accelerate research in this area. These will be incorporated into the next iteration of the National Plan and will be updated on an annual basis with the assistance of consensus advice from the Advisory Council.
(ONGOING) Action 1.A.4: Update research priorities and milestones
Lead Agency: ASPE
Partners: NAPA Advisory Council, NIH/NIA
To ensure that the research priorities and milestones reflect the broad input of the scientific community and the public, one Advisory Council meeting per year will be focused on this area. The Research Subcommittee of the Advisory Council will collect input and recommend priorities and milestones for consideration by the Advisory Council as official recommendations. As appropriate, researchers in the field will also be invited to present at these meetings.
(ONGOING) Action 1.A.5: Create a timeline with milestones for achieving Goal 1
Lead Agencies: NIA, NINDS
Since the advent of the National Plan, NIH’s planning process for research on AD/ADRD has expanded in inclusion and scope among NIH Institutes and Centers and stakeholders across the scientific and care communities. Hearing a diverse expertise and opinions is critical to updating research recommendations based on an open review of scientific progress. It also ensures prioritization based on important scientific questions that must be answered to advance our understanding of these complex disorders and helps identify how federal and other public and private organizations can most effectively collaborate to address research priorities. Ultimately, information obtained through the various research summits results in the formation and/or update of the implementation research milestones, which set forth activities through FY 2025 to address the goals of the National Plan. The latest of these updates took place after the Alzheimer’s Disease Research Summit in April 2018 and Alzheimer’s Disease-Related Dementias Summit in March 2019. Updates are in process following the 2020 Care Summit and the 2021 Alzheimer’s Disease Research Summit.
For more information, see:
- https://www.nia.nih.gov/research/milestones
- https://www.nia.nih.gov/sites/default/files/2018-07/fy2020-milestones-chart.pdf
(ONGOING) Action 1.A.6: Regularly convene an Alzheimer’s disease-related dementias summit to review progress on research recommendations, and refine and add new recommendations as appropriate based on recent scientific discoveries
Lead Agency: NINDS
Partners: academia, industry, professional and advocacy groups
The National Institute of Neurological Disorders and Stroke (NINDS) convened the most recent ADRD Summit on March 14-15, 2019. The next ADRD Summit will take place in March 2022, and initial planning is underway. As in the past, researchers, clinicians, patients, caregivers, families, and advocates will gather to assess scientific progress and update research recommendations for the ADRD scientific communities including a special focus on mixed dementias, VCID, FTD, and LBD as well as the broader cross-cutting areas, such as AD/ADRD health disparities.
For more information, see:
- https://www.ninds.nih.gov/sites/default/files/2019_adrd_summit_recommendations_508c.pdf
- https://aspe.hhs.gov/alzheimers-disease-related-dementias-adrd-summit-2019-prioritized-research-milestones
- https://www.ninds.nih.gov/News-Events/Events-Proceedings/Events/Alzheimers-Disease-Related-Dementias-Summit-2019
(UPDATED) Action 1.A.7: Regularly convene a Research Summit on Care, Services, and Supports for Persons with Dementia and their Caregivers
Lead Agencies: ASPE, NIH
Partners: NAPA Advisory Council, academia, industry, professional and advocacy groups
Following the success of the first Summit in 2017, the second National Research Summit on Care, Services, and Supports for Persons with Dementia and their Caregivers, hosted and sponsored by the National Instituteon Aging (NIA) with support from contributors through the Foundation for the NIH, was held as a Virtual Summit Series in 2020.
The 2020 Care Summit brought together individuals representing a variety of disciplines and backgrounds, including researchers as well as those living with dementia, care partners, providers, and advocates to identify evidence-based programs, strategies, approaches, and other research that can be used to improve the care, services, and support of persons living with dementia and their caregivers. Released in December 2020, the final report summarizes research gaps and opportunities for propelling advances in policy, practice, and care. These include the need for research on the economic impact of care on individuals, families, health systems, and society; and the need for innovation in how medical care and LTSS for persons with dementia are organized, financed, and delivered. There is strong evidence of profound disparities in dementia care among subpopulations most affected by AD/ADRD, and new research is needed to explore effects on health and receipt of care in subpopulations that are less well understood (e.g., minoritized populations and those who live alone with dementia).
For more information, see:
- https://www.nia.nih.gov/2020-dementia-care-summit
- https://www.nia.nih.gov/2020-dementia-care-summit#Materials
- https://www.nia.nih.gov/research/summit-gaps-opportunities
- https://twitter.com/search?q=%23DementiaCareSummit&src=typeahead_click&f=live
- https://www.nia.nih.gov/sites/default/files/2021-01/DementiaCareSummitReport.pdf
(ONGOING) Action 1.A.8: Regularly review the Congressionally Directed Medical Research Program’s Peer Reviewed Alzheimer's Research Program Strategic Plan
Lead Agency: DoD
The Congressionally Directed Medical Research Program (CDMRP) is a partnership between the U.S. Congress, the military, and the public to fund innovative and impactful research in targeted program areas. One of the CDMRP’s is the Peer Reviewed Alzheimer's Research Program (PRARP), which is specifically focused on understanding the relationship between traumatic brain injury (TBI) and dementia. In 2019, the PRARP released an updated Strategic Plan that identified the high-impact research goals in the areas of TBIs and AD/ADRD. The Strategic Plan summarizes research funding and findings though the PRARP program since 2011, and identified short, medium, and long-term goals for the program.
For more information, see:
Strategy 1.B: Expand Research Aimed at Preventing and Treating Alzheimer’s Disease and Related Dementias
HHS and its federal partners will expand clinical trials on pharmacologic and non-pharmacologic interventions to prevent AD/ADRD and manage and treat its symptoms. The Federal Government will address the challenge of enrolling people in clinical trials who are representative of the country’s diverse population, including racial and ethnic groups that are at higher risk for AD/ADRD, through new partnerships and outreach. These actions will build on ongoing research focused on the identification of genetic, molecular, and cellular targets for interventions and build on recent advances in the field.
(UPDATED) Action 1.B.1: Expand research to identify the molecular and cellular mechanisms underlying Alzheimer’s disease and related dementias, and translate this information into potential targets for interventions
Lead Agencies: NIA, NINDS
Partners: potential research partners in the public and private sectors
In the past year, NIA and NINDS have issued several funding opportunity announcements (FOAs) focused on research to help develop a better understanding of the growing list of genetic risk factors and molecular pathways that are involved in AD/ADRD. For example, in 2020, under the “Molecular Mechanisms of Blood Brain Barrier Function and Dysfunction in AD/ADRD” FOA, NINDS funded four large research teams to examine how damage to the blood brain barrier occurs and how it may contribute to cognitive impairment. In response to these FOAs and investigator-initiated studies, researchers are developing a new generation of research tools to identify, explore, and validate a variety of targets with therapeutic potential. These sophisticated tools allow researchers to collect and integrate layers of biological data in novel ways, opening the door to new insights into the origins and progression of AD/ADRD.
These new tools are also helping researchers gain a clearer picture of the complex underlying mechanisms of these devastating neurological disorders. They are leading to an understanding of the interplay among relevant molecules and systems, the relationship between amyloid and tau proteins, the role of immunity and inflammation, the involvement of metabolic and cardiovascular pathways, the regulation of cell-type-specific proteome dynamics, the characterization of the preclinical/prodromal phase of a-synucleinopathies, the etiology of infectious pathogens in AD/ADRD, and the selective cell and network vulnerability and impact of brain aging in neurodegenerative diseases. This broader view of the basic biology of AD/ADRD could lead to potential breakthroughs. One type of tool that is critical for understanding what may be happening in the brains of patients is animal models. To fill the critical need for next-generation animal models for AD/ADRD, NINDS recently awarded several large grants under the Development and Validation of Advanced Mammalian Models for AD/ADRD FOA in order to develop models for FTD, VCID, LBD, and mixed dementias/neurodegeneration.
For more information, see:
- https://grants.nih.gov/grants/guide/pa-files/PAR-19-070.html
- https://grants.nih.gov/grants/guide/pa-files/PAR-19-071.html
- https://grants.nih.gov/grants/guide/pa-files/PAR-19-338.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-20-016.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-21-013.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-21-029.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-21-033.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-21-034.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-18-046.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-18-048.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-18-051.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-18-052.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-19-007.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-19-033.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-21-016.html
- https://grants.nih.gov/grants/guide/pa-files/PAS-19-317.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-MH-19-510.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-MH-19-511.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-19-013.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-19-015.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-19-026.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-19-027.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-19-030.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-19-039.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-20-004.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-20-005.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-21-003.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-21-006.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-21-007.html
- https://reporter.nih.gov/search/14EBCE084D84C2D07598B8961CAA4A01A2FFCEB861BF/projects?shared=true&legacy=1
A key part of NIH’s strategy for developing new treatments for AD/ADRD is to bolster the translation of basic research findings into discovery and development of new drugs and devices for disease treatment and prevention. The length of time required for researchers to discover a biological mechanism of disease, such as a gene variant that does not function normally, and then develop an effective treatment without toxic side effects has been 12-15 years. Additionally, very few drug candidates or devices succeed through the pipeline to reach FDA approval, because they are not found to be both safe and effective. To accelerate the discovery of effective treatments that will become broadly available to the public, NIH has developed programs to make data, knowledge, and research tools widely available to all researchers. Instead of competing with each other, stakeholders in industry, academia, and government are collaborating to reach a common goal: developing effective treatments for AD/ADRD.
For more information, see:
- https://grants.nih.gov/grants/guide/pa-files/par-18-820.html
- https://grants.nih.gov/grants/guide/pa-files/par-19-146.html
- https://grants.nih.gov/grants/guide/pa-files/par-19-147.html
- https://grants.nih.gov/grants/guide/pa-files/PAR-20-110.html
- https://grants.nih.gov/grants/guide/pa-files/PAR-20-122.html
Thanks to the substantial investment in AD/ADRD research over the past several years, NIH has increased its drug discovery efforts significantly. Of the many therapeutic programs supported by NIH for AD/ADRD, ten have now matured through the preclinical development process and are currently being tested in humans in Phase I and Phase II clinical trials. These ten new drug candidates target multiple aspects of the disease process including neuroinflammation, proteostasis (e.g., abnormal protein folding), neurogenesis, synaptic dysfunction, etc.
Established in 2019, TaRget Enablement to Accelerate Therapy development for Alzheimer’s Disease (TREAT-AD) consortium is another recent addition to NIH-supported translational infrastructure established through the Alzheimer’s Centers for Discovery of New Medicines. This $73 million enterprise has two translational centers with a common mission: to diversify and accelerate therapy development for AD/ADRD through the development of open-source tools, reagents, and methods for robust validation of candidate targets delivered by the Accelerating Medicines Partnership® Program for Alzheimer’s Disease (AMP®-AD) program and other target discovery programs and by integrating a set of novel targets into drug discovery campaigns. Each TREAT-AD center brings together world-class expertise in data science, computational biology, disease biology, structural biology, assay development, medicinal chemistry, pharmacology, and clinical research.
For more information, see:
NIH’s Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs are an integral source of capital for early-stage United States small businesses that are creating innovative technologies to improve health. These programs help small businesses break into the federal research and development arena, create life-saving technologies, and stimulate economic growth. This funding also helps the private sector bring promising technologies to the consumer market. Through these programs, NIH is leveraging the economic engine of small businesses to enhance scientific innovation. Before the increased funding for AD/ADRD (2010-2013), NIA awarded 73 AD/ADRD SBIR/STTR grants to 59 small companies. After the increased appropriations (2017-2020), NIA approximately tripled that achievement by awarding 235 AD/ADRD SBIR/STTR grants to 168 companies for discovery and development of new treatments as well as biomarker research and technologies for improving care and caregiving. In the past year, NIA partnered with the Administration for Community Living (ACL) to publish RFA-AG-21-025 - Development of Cost-Effective and Customizable Training and Education Platforms for AD/ADRD Caregivers that Focus on Addressing Financial Management and Legal Planning (R43/R44 Clinical Trial Not Allowed) for which seven awards were made.
For more information, see:
- https://grants.nih.gov/grants/guide/pa-files/pas-19-316.html
- https://grants.nih.gov/grants/guide/pa-files/pas-19-317.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-21-025.html
NIH’s AMP-AD and AMP Program for Parkinson’s Disease (AMP®-PD) programs have transformed the way that data and biological samples are shared freely, biological targets are discovered, and drug candidates are chosen and developed. NIH recently announced the next version of the AMP-AD program (2.0). During the first phase, the AMP-AD program’s open science, big data approach enabled research teams to identify and make publicly-available more than 500 unique candidate targets for this complex disease. In the second phase, NIH is leading research efforts to enable a precision medicine approach to discovery of novel therapeutic targets and biomarkers. AMP-AD will also focus on generating data from diverse cohorts, specifically Black and Latino cohorts who are disproportionately affected by the disease. All data and analytical tools will be made available to the wider research community through a centralized data infrastructure, the AD Knowledge Portal. The AD Knowledge Portal also serves as the central repository for the other NIH open science target discovery consortia, Molecular Mechanisms of the Vascular Etiology of Alzheimer's Disease (M2OVE-AD), Resilience-AD, and Psych-AD.
For more information see:
- https://www.nih.gov/research-training/accelerating-medicines-partnership-amp
- https://www.nia.nih.gov/research/amp-ad
- https://www.ninds.nih.gov/Current-Research/Focus-Disorders/Accelerating-Medicines-Partnership-Parkinsons-Disease-AMP-PD
- https://www.nia.nih.gov/research/dn/alzheimers-disease-sequencing-project-study-design
- https://www.nia.nih.gov/research/blog/2020/11/open-science-delivers-wealth-ad-adrd-research-data-portal-near-you
- https://www.nia.nih.gov/news/nih-invests-next-iteration-public-private-partnership-advance-precision-medicine-research
NIA recently funded several new projects through its Alzheimer’s Drug Development Program. Each project is focused on developing a new drug that targets a different biological process, such as brain inflammation, known to go awry during the development of AD/ADRD. If successful, these NIH-supported preclinical drug development studies will result in new candidate drugs that could then be tested in people.
For more information, see:
In 2020, NIH broke ground on its Bethesda, Maryland, campus to construct a new intramural research facility devoted to AD/ADRD research. This Center for Alzheimer’s Disease and Related Dementias (CARD) will support basic, preclinical, and clinical research. Center initiatives will complement and enhance the work of thousands of researchers nationwide and beyond who are exploring disease mechanisms to translate scientific knowledge into ways to better prevent and treat these diseases. While the dedicated facility is expected to fully open its doors in early 2022, CARD researchers are already building multi-disciplinary collaborations among scientists on the NIH campus and in academia and industry.
For more information see:
NIH provides new animal models for basic research or for therapy development. For example, to date, research teams that are part of the NIA-supported Model Organism Development and Evaluation for Late-Onset Alzheimer’s Disease (MODEL-AD) consortium have created more than 50 genetically modified mouse models. These mice are available to the research community through the Jackson Laboratory Center for Alzheimer’s and Dementia Research’s Mouse Model Resource, and the data, protocols, and other resources are available through the AD Knowledge Portal.
For more information see:
- https://www.model-ad.org/
- https://adknowledgeportal.synapse.org/
- https://adknowledgeportal.synapse.org/Explore/Experimental%20Tools
NIH’s ability to quickly respond to the urgent need for vaccine and treatment development for the coronavirus pandemic was made possible through experience from its already-established open science initiatives such as the AMP-AD and AMP-PD programs, M2OVE-AD, Resilience-AD, Psych-AD, MODEL-AD, and TREAT-AD. These and similar NIH initiatives have transformed the way that scientists collaborate rather than compete, share their data and biological samples, work together to discover new biological mechanisms of disease, and find new drug candidates for testing.
For more information see:
- https://www.nia.nih.gov/research/amp-ad
- https://www.nia.nih.gov/research/blog/2020/11/open-science-delivers-wealth-ad-adrd-research-data-portal-near-you
- https://www.model-ad.org/
- https://www.nia.nih.gov/news/nih-funded-translational-research-centers-speed-diversify-alzheimers-drug-discovery
One way that NIH works to find effective ways to treat dementia is by considering drugs that FDA has already deemed safe for people with other conditions. The NIA Intramural Research Program has recently launched the Drug Repurposing for Effective Alzheimer’s Medicines (DREAM) study. DREAM is a collaboration with researchers at Harvard Medical School, Rutgers University, and Johns Hopkins University School of Medicine to repurpose FDA-approved drugs for treatment of dementia. NIA also funds drug repurposing research at its grantee institutions. NIH released a funding initiative in 2020 called Translational Bioinformatics Approaches to Advance Drug Repositioning and Combination Therapy Development for Alzheimer’s Disease, which aims to leverage the power of big data and open science in advancing drug repurposing and combination therapy development.
For more information see:
- https://pubmed.ncbi.nlm.nih.gov/33304987/
- https://www.nia.nih.gov/news/nia-study-identifies-fda-approved-drugs-may-also-be-helpful-dementia
- https://grants.nih.gov/grants/guide/pa-files/PAR-20-156.html
NIA supports the Alzheimer’s Disease Preclinical Efficacy Database (AlzPED), which plays a role in creating a road map towards increased rigor and reproducibility in preclinical AD/ADRD studies. AlzPED, a joint project of NIA, the NIH Library, the Alzheimer’s Association, and the Alzheimer’s Drug Discovery Foundation, is a publicly-available, searchable knowledge database which hosts 1,000+ studies on preclinical testing of candidate therapeutics for AD/ADRD.
For more information, see:
The AD Knowledge Portal, an informatics data-sharing platform that began as the data repository for the AMP-AD Target Discovery Program, and the portal-linked, open-source platform Agora have enabled access to a vast amount of high-quality molecular data, analytical results, and candidate targets generated by the AMP-AD program research teams. The AD Knowledge Portal now includes data and resources from other NIA-supported team-science projects operating under open science principles.
For more information, see:
NIA’s Small Research Grant Program for the Next Generation of Researchers in AD/ADRD Research Program is designed to encourage a next generation of scientists to pursue research and academic careers in neuroscience, AD/ADRD, and healthy brain aging. NIA seeks to turn fresh ideas from scientists in other fields into pilot studies for innovative AD/ADRD research programs that leverage and build upon their existing expertise and to build a more robust pipeline of committed AD/ADRD researchers.
For more information, see:
- https://grants.nih.gov/grants/guide/pa-files/PAS-19-391.html
- https://grants.nih.gov/grants/guide/pa-files/PAS-19-392.html
- https://grants.nih.gov/grants/guide/pa-files/PAS-19-393.html
(UPDATED) Action 1.B.2: Expand genetic epidemiologic research to identify biological and genetic risk and protective factors for Alzheimer’s disease and related dementias
Lead Agencies: NIA, NINDS
Partners: research partners in the public and private sectors
Another key component in the growing toolkit of precision medicine for AD/ADRD is the Alzheimer's Disease Sequencing Project (ADSP), an international resource of genetics data from multiple centers and studies. Launched in 2012, the ADSP is designed to promote innovative collaboration among scientists to provide genetic samples for sequencing with the goal of identifying from multi-ethnic populations new genetic variants that influence risk and protection from AD/ADRD. This project involves more than 150 international investigators at 33 institutions. Data come from more than 60 cohorts of research participants. The Genome Center for Alzheimer’s Disease quality control checks and harmonizes all of the genetic data so that when a variant in the genome is uncovered, it can be compared against the data from thousands of other genomes. The NIA Genetics of Alzheimer’s Disease Data Storage Site (NIAGADS) serves as the ADSP Data Coordinating Center. In 2017, NIA launched the ADSP Follow-Up Study, and in 2021 the Follow-Up Study 2.0. Together, these initiatives aim to pursue rare variants in a range of different populations (e.g., Black, Hispanic, American Indian/Alaska Native [AI/AN], Asian). Teams are presently working to recruit new cohorts of ethnically diverse participants.
In keeping with the high priority that the AD/ADRD genetics community places on diversity, the ADSP plans to have more than 100,000 ethnically diverse study participants by 2023. An important overarching goal of the ADSP Follow-Up Study is to genetically define sub-groups of subjects that carry specific sets of genes and match them with biomarkers, functional genomics, and clinical data. This will define subtypes of the disease. Defining subtypes will allow better selection of subjects for clinical trials because outcomes of drug therapies can be better targeted toward groups of individuals who have similar characteristics. It is particularly important to define ethnic diversity in terms of disease risk because ethnic groups vary widely in the degree of risk at particular locations in the genome and it is likely the clinical trials will need to be designed differently depending upon the ethnicity of the study population.
The 2021 Phenotypic Data Harmonization Initiative is harmonizing clinical data from all of the ethnic cohorts in the ADSP. These data will become a long-lived “legacy” dataset that will be perpetually curated. A network of researchers with expertise in genetics, epidemiology, and clinical specialties are working with the ADSP and with study cohort leads on data harmonization efforts to optimize the ability to identify well-targeted therapeutic approaches for AD/ADRD. The National Alzheimer’s Coordinating Center (NACC) shares phenotypic and related clinical data with the ADSP and is strongly supporting this initiative.
The ADSP also recently launched a Machine Learning/Artificial Intelligence initiative. The amount of genetic data that now is available is massive and it has been extraordinarily difficult to analyze using classical methods because the data are so complex. This initiative supports the development of fast and efficient Machine Learning/Artificial Intelligence approaches to identify the genetics that increase risk of or protection against AD/ADRD. The emphasis is on the development and sharing of transformative Machine Learning/Artificial Intelligence-based systems, emerging tools, and modern technologies for the analysis of genetic data.
In 2021, the ADSP also launched a Functional Genomics consortium. Functional interpretation of genetic variations has been challenging historically and remains a persistent bottleneck in genetic studies of complex diseases. This hinders the discovery of genetic-based targets for therapeutics. To connect genetic variants to downstream effectors and functions, a number of issues will be addressed by this initiative, including the need to: (1) pinpoint causal variants that affect disease susceptibility and/or progression; (2) characterize the molecular and biochemical effect of these variants and identify the target genes on which these variants act and the cell-types and states in which these variants operate; (3) determine links to heterogeneous cellular and pathologic mechanisms; and (4) identify genetic drivers underlying AD endophenotypes that are clinically relevant but difficult to ascertain. Investigators from the AMP-AD program and ADSP Consortia are working together to find intersections between the gene clusters that the ADSP has identified and the functional networks that the AMP-AD program team has reported.
NIAGADS now hosts 74 human genetics datasets with 90,743 samples and has a genomics database for cross-referencing and visualizing known genomic variants. All data generated by the ADSP are deposited into NIAGADS. As of August 2020, NIAGADS has shared 16,906 whole-genomes and 20,504 whole-exomes to the research community and anticipates sharing an additional 30,000 whole-genomes by the end of the year. Using data from NIAGADS and other repositories, scientists have been able to expand the number of known genetic risk factors for AD/ADRD, and several others are under investigation.
The National Centralized Repository for Alzheimer’s Disease and Related Dementias (NCRAD) is an NIA-supported resource to help scientists accelerate and streamline their efforts. NCRAD serves AD/ADRD scientists by banking a wide range of biospecimens, recently including pluripotent stem cells. Through a collaboration with NIAGADS, NCRAD supports state-of-the-art genome and genotyping arrays for samples in several new studies, including the 90+ Study, a longitudinal study of aging and cognition among participants over age 90, and the Amyloid Neuroimaging and Genetics Initiative, an add-on for participants in the Imaging Dementia-Evidence for Amyloid Scanning Study.
For more information, see:
- https://grants.nih.gov/grants/guide/pa-files/par-19-070.html
- https://grants.nih.gov/grants/guide/pa-files/par-19-071.html
- https://grants.nih.gov/grants/guide/pa-files/PAR-19-234.html
- http://grants.nih.gov/grants/guide/pa-files/PAR-19-269.html
- https://grants.nih.gov/grants/guide/pa-files/PAR-19-288.html
- https://grants.nih.gov/grants/guide/pa-files/par-20-099.html
- https://grants.nih.gov/grants/guide/pa-files/PAR-20-110.html
- https://grants.nih.gov/grants/guide/pa-files/PAR-21-212.html
- https://grants.nih.gov/grants/guide/rfa-files/rfa-ag-21-005.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-21-006.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-22-001.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-22-021.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-20-021.html
- https://www.niagads.org/
- https://www.niagads.org/adsp/content/home
- https://www.nia.nih.gov/research/resource/national-centralized-repository-alzheimers-disease-and-related-dementias-ncrad
In addition to ADSP, NIA has several ongoing FOAs that call for research to enhance the ability to uncover the genetic underpinnings of AD/ADRD, furthering our understanding of rare risk and protective variants. Today, thanks in part to the increased investment in AD/ADRD research, scientists have identified variants in more than 50 regions of the genome that may increase risk for the disease. Of these, variants in more than 23 individual genes have been linked to increased risk of late-onset Alzheimer’s disease (LOAD). These genetic regions appear in clusters that point toward what may be highly relevant molecular pathways. By understanding key pathways, researchers may be able to develop prevention strategies and treatments for AD/ADRD.
For more information, see:
- https://grants.nih.gov/grants/guide/pa-files/PAR-18-889.html
- https://grants.nih.gov/grants/guide/pa-files/PAR-19-269.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-18-001.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-18-046.html
To advance further discovery for genetic factors and molecular pathways involved in FTD, NIH is also supporting the FTD Sequencing Consortium. This genetics consortium is composed of researchers at universities in the United States and at NIH who are utilizing whole-genome sequence technology to generate sequence for 4,000 autopsy-confirmed and clinical characterized FTD cases.
For more information, see:
- https://grants.nih.gov/grants/guide/notice-files/NOT-NS-18-082.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-17-017.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-21-003.html
- https://www.allftd.org/
NINDS continues to support new initiatives focused on identifying vascular risk profiles that may predict cognitive decline and dementia. For example, in late 2019 NINDS launched Determinants of Incident Stroke Cognitive Outcomes and Vascular Effects on Recovery, a large 6-year prospective clinical research study which aims to determine the specific subsets of stroke events that cause and do not cause cognitive impairment and dementia in post-stroke populations. In 2020, NINDS launched “Diverse VCID: White Matter Lesion Etiology of Dementia in Diverse Populations,” which is a consortium supporting 27 investigators at 12 institutions to conduct clinical research using MRI and other measures to determine how white-matter lesions contribute to cognitive impairment and dementia. Both of these research programs include a special focus on racial and ethnic populations that experience dementia health disparities, as well as elucidating what additional clinical factors and co-morbidities synergize with stroke to result in cognitive impairment and dementia outcomes.
For more information, see:
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-19-013.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-20-013.html
In 2021, U.S. Department of Veterans Affairs (VA) provided supplemental funds to studies that curate and develop AD phenotypes using VA clinical data. These studies would produce pilot data for VA to contribute to collaboration with the NIA.
(UPDATED) Action 1.B.3: Increase enrollment in clinical trials and other clinical research through community, national, and international outreach
Lead Agency: NIA
Partners: ACL, FDA, VA, CDC, HRSA
Starting in 2016, with facilitation by the Alzheimer’s Association and in close collaboration with experts from government, private, and academic sectors, NIA led an effort to develop comprehensive goals and strategies to enhance recruitment into clinical research, particularly focusing on underrepresented communities. To ensure broader input, NIA gathered feedback on the recruitment strategies through the IdeaScale crowdsourcing platform. These efforts resulted in the National Strategy for Recruitment and Participation in Alzheimer’s Disease and Related Dementias Clinical Research.
For more information, see:
In 2019, NIA launched Alzheimer’s and Dementia Outreach, Recruitment, and Engagement Resources (ADORE), a searchable collection of materials designed to support recruitment and retention into clinical trials and studies. ADORE supports the National Strategy and represents some of the materials and activities that Alzheimer's Disease Research Centers (ADRCs), Alzheimer’s Clinical Trials Consortium (ACTC), NIA and the broader NIH, and other organizations have developed to engage people in research. In addition, NIA developed several collateral materials to include in ADORE, including a recruitment planning guide, a series of testimonial videos, and an easy-to-read booklet to promote older adult research participation. The repository has evolved as researchers have nominated their resources for NIA’s consideration. Newly added resources include a brain donation Q&A web page, an infographic on the difference between clinical trials and observational studies, tools to reduce disparities in research participation among Asian Americans and Pacific Islanders (AAPI), a dementia-friendly toolkit, educational and recruitment videos, a brain health guide, and a research participant Q&A flyer.
NIA recently released a web‐based communication tool, called Outreach Pro, that will enable health care professionals in the community to easily produce a “package” of tailored materials and strategies that can be branded locally to increase participant recruitment for clinical studies. NIA conducted focus groups, surveys, and stakeholder interviews to tailor recruitment materials for clinical studies to reach underrepresented populations more effectively. Using the findings from this research, NIA in 2020 developed a set of materials and messaging, including videos and other multi-media, print ads, posters, and social media, tailored to Spanish-speaking communities and available in both English and Spanish. A similar approach was used in 2019 to develop materials for Black audiences. Outreach Pro allows the research community to access, adapt, and personalize these materials for underrepresented communities. Outreach Pro launched in Summer 2021.
For more information, see:
- https://www.nia.nih.gov/research/adore
- https://www.nia.nih.gov/research/alzheimers-dementia-outreach-recruitment-engagement-resources
- https://outreachpro.nia.nih.gov
NIA continues to promote participation in AD/ADRD clinical trials, studies, and registries through Alzheimers.gov and its Alzheimer’s Disease Education and Referral (ADEAR) website portal; clinical trials listing and monthly e-alert to more than 26,000 subscribers; social media; infographics; presentations; promotion of ADORE materials; and collaboration with other federal agencies and advocacy organizations to encourage research participation among older adults, including through the Focus on Aging interagency webinar series. All materials are drafted in plain language formats for ease of communications.
For more information, see:
In 2020, NIA collaborated with the Alzheimer’s Association to host the Developing Applied Science of Recruitment and Retention for Alzheimer’s Disease and Related Dementias Clinical Research Symposium at the 2020 Alzheimer’s Association International Conference (AAIC). This virtual symposium provided a broad perspective that builds on existing scientific knowledge to support the goal of ultimately accelerating and expanding research efforts on recruitment strategies for clinical trials.
For more information, see:
A key factor for improving enrollment is to help researchers monitor actual recruitment against planned milestones. To achieve the ability to track, report, and manage enrollment data, NIA is developing and will soon launch a unified Clinical Research Operations and Management System (CROMS). Through CROMS, NIA will track, manage, and report enrollment data and activities made possible via the NIA-funded clinical research portfolio. CROMS will provide critical and real-time information to ensure that NIA-supported clinical studies are making appropriate progress toward reaching their inclusion recruitment goals related to multiple underrepresented groups.
For more information, see:
Through the Examining Diversity, Recruitment, and Retention in Aging Research funding opportunity, NIA awarded support for several projects focused on improving research tools, methods, and recruitment practices.
For more information, see:
Since 2020, the VA has been one of the recruitment networks for the NIA-funded Pragmatic Evaluation of Events and Benefits of Lipid-lowering in Older Adults trial, which aims to determine whether statin can prevent dementia and disability in addition to heart disease and other cardiovascular-related deaths. The VA Cooperative Studies Program (CSP) Pharmacy Coordinating Center serves as the central pharmacy for the trial to distribute medications to study participants.
For more information, see:
- https://www.defensemedianetwork.com/stories/va-research-the-va-and-clinical-trials-veterans-affairs/
- https://www.trialsitenews.com/nia-invests-90m-into-preventable-study-determining-the-benefits-of-statins-atorvastatin-in-the-elderly-with-duke-and-wake-forest-leading-the-effort/
- https://dcri.org/preventable/
In 2019, the Health Resources and Services Administration’s (HRSA’s) Geriatrics Workforce Enhancement Program (GWEP) Notice of Funding Opportunity included language calling for applicants to describe how they would educate and train patients, families, caregivers, direct care workers, health care providers, and health professions students, faculty, residents, and fellows on when it is appropriate to recruit older adults into research. This training continues into the second year of funding (FY 2020).
For more information, see:
(UPDATED) Action 1.B.4: Monitor and identify strategies to increase enrollment of racial and ethnic minorities in Alzheimer’s disease and related dementias studies
Lead Agencies: NIA, NIMHD
Partner: ACL
See Action 1.B.3 for updates regarding the National Strategy for Recruitment and Participation in Alzheimer’s Disease Clinical Research released in Fall 2018. This strategy includes approaches to increase enrollment of racial and ethnic minorities in AD/ADRD studies as recommended by the National Strategy Group’s Local, Diverse Working Group and outlined in the Alzheimer’s Disease and Related Dementias Clinical Studies Recruitment Planning Guide.
For more information, see:
- https://www.nia.nih.gov/research/recruitment-strategy
- https://www.nia.nih.gov/sites/default/files/2019-05/ADEAR-recruitment-guide-508.pdf
In April 2018, NIA released a new FOA -- Examining Diversity, Recruitment and Retention in Aging Research -- to encourage building new, collaborative teams to target gaps in recruitment and retention methods and outcomes, as well as to establish the community infrastructure needed to accelerate recruitment. Another FOA encourages applications that examine mediators of disparities in AD/ADRD, using diverse cohorts of subjects with a focus on strategies for recruitment and retention in clinical trials. In addition to disparities-focused initiatives, NINDS has now issued several clinical research FOAs which require investigators to apply their research questions to at least two populations of study. Another initiative invites applications for sample acquisition, genome-wide association studies, whole-genome sequencing, quality control checking, variant calling, data calling, data-sharing, data harmonization, and analysis that will support the generation of data from multi-ethnic cohorts for the ADSP Follow-Up Study 2.0. These grant activities are ongoing.
For more information, see:
- https://grants.nih.gov/grants/guide/pa-files/PAR-18-749.html
- https://grants.nih.gov/grants/guide/pa-files/PAR-21-212.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-17-012.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-19-012.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-20-013.html
In 2021, NIA provided 1-year supplemental funds to five VA sites to facilitate subject recruitment to NIA-funded studies.
Additionally, the National Institute on Minority Health and Health Disparities (NIMHD) began a new clinical trial on “Addressing the Knowledge and Recruitment Gap in Alzheimer’s Disease and Precision Medicine among Native People”. This study will evaluate recruitment strategies for AI/AN and proposes several specific aims: create culturally-appropriate materials on Alzheimer’s Disease and Precision Medicine (AD-PM) (Phase 1); evaluate the clarity and acceptability of the materials and their effect on completion of the AD-PM Module in a randomized controlled trial and subsequent enrollment into an AD-PM cohort (Phase 2); identify patient-level predictors of enrollment; and evaluate potential differences in the effectiveness of recruitment approach by age, sex, education, cultural identity, and rurality (Phase 2).
For more information, see:
- https://projectreporter.nih.gov/project_info_description.cfm?aid=9570695&icde=41152371&ddparam=&ddvalue=&ddsub=&cr=2&csb=default&cs=ASC&pball=
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-18-047.html
- https://projectreporter.nih.gov/Reporter_Viewsh.cfm?sl=15EEC10D4C8DC5D37598B8961CAA4A01A2FFCEB861BF
The NIA-supported ACTC aims to develop and implement cutting-edge participant recruitment and retention strategies, especially in diverse populations, and to establish a new Minority Outreach and Recruitment Team. This network of clinical trials with 35 United States sites will develop, harness, and deploy the best practices and latest methods for the conduct of AD/ADRD trials.
For more information, see:
NIA also supports 33 ADRCs at major medical institutions across the United States. Researchers at these ADRCs are working to translate research advances into improved strategies for prevention, diagnosis, treatment, and care for people living with AD/ADRD. Although each ADRC has its own area of emphasis, these ADRCs also enhance research on AD/ADRD via a network approach that encourages the exchange of new research ideas and approaches as well as data, biological samples, and genetic information. The ADRCs also enhance and promote diversity of research participants. For example, the ADRCs have set up a Hispanic interest group that includes a Listserv for Hispanic researchers, those with an interest in research with Hispanic participants, and issues specific to Spanish language assessment. This group is helping to ensure that materials are available in Spanish, thereby addressing the needs of Spanish speaking participants, and to assure research capacity (with both materials and staff training) for assessment in Spanish. In addition to Spanish, assessments at ADRCs have also been translated into Chinese.
To further incentivize innovative ideas and opportunities in AD/ADRD research, NIA has funded four exploratory ADRCs. These new centers will broaden current ADRC research initiatives with underrepresented populations such as Black Americans, Native Americans, and those in rural communities -- all of which have different risk factors for developing these devastating diseases.
See Action 1.B.3 for information on Outreach Pro, a web-based tool that will enable health care professionals in the community to easily produce a “package” of tailored materials and strategies that can be branded locally to increase participant recruitment for clinical studies. Designed to help reach multiple cultures and those who do not speak English, the tool was launched in July 2021. It enables the research community to access, adapt, and personalize the materials that NIA has developed for underrepresented communities.
NIA has been conducting focus groups, surveys, and stakeholder interviews to tailor recruitment materials for clinical studies to reach underrepresented populations more effectively. Using the findings from this research, NIA has developed a set of materials and messaging, including videos and other multi-media, print ads, posters, and social media, tailored to diverse populations, including Black and Hispanic, in both English and Spanish. Currently, materials are in development for Chinese Americans, Indian Americans, and Filipino Americans in both English and their respective languages. In 2022, NIA will use a similar approach to develop materials for AI/AN. NIA has also recently developed a Spanish version of the Alzheimers.gov website. All materials developed are available to the public in both ADORE and Outreach Pro.
NIA recently funded Foundations of Representative Engagement, Valid, and Effective Recruitment in Alzheimer’s Research. Through this project, researchers are developing and implementing novel methods for recruitment, engagement, and retention of minorities into AD/ADRD studies through community engagement and the ADRCs. The research team is also developing recruitment, engagement, and retention metrics and interventions and establishing communications frameworks to improve literacy for both the general public and research communities.
For more information, see:
- https://www.nia.nih.gov/research/adc
- https://www.nia.nih.gov/news/nih-expands-nations-alzheimers-and-related-dementias-research-capacity
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6249084/
- https://reporter.nih.gov/search/ixuI3REUAEeJqoU8Xa4hOA/project-details/10094911#description
- https://outreachpro.nia.nih.gov
- https://www.alzheimers.gov/es?utm_source=partner-eblast&utm_medium=affiliate&utm_campaign=alzesp-202110&utm_term=NAPA
In April 2021, NIA hosted a virtual meeting to discuss the potential and planning of a practice-based research network (PBRN) to address the disparities gap with the recruitment and retention of diverse and under-served populations in AD/ADRD clinical research studies. PBRNs are networks of health care clinicians and practices working together to answer community-based health care questions, to translate research findings into practice, and to directly engage diverse and under-served communities in AD/ADRD clinical research. NIA is investigating the possibility of developing a PBRN as a long-term solution to create sustainable and mutually beneficial relationships with under-served communities to address the systemic barriers that reduce their potential to participate in AD/ADRD and aging clinical research studies. Since the meeting, NIA has been working to organize an external group of researchers and community organizations to offer input, feedback, and recommendations on how to develop a successful AD/ADRD PBRN. A subsequent step will be to identify potential PBRN models and pilot them in communities to assess feasibility and impact on clinical study participation as well as community engagement around participation.
For more information, see:
- https://impactcollaboratory.org/event/nia-virtual-meeting-development-of-an-nia-practice-based-research-network-to-conduct-ad-adrd-clinical-research/
- https://videocast.nih.gov/watch=41795
(ONGOING) Action 1.B.5: Conduct clinical trials on the most promising pharmacologic interventions
Lead Agency: NIA
Partner: VA
Most of the NIH-supported drug trials for AD/ADRD are in an early stage, which means Phase 1 or Phase 2 trials, but several Phase 3 trials are also in progress. With each successive phase, a longer period of time and more participants are needed to conduct the study. A number of NIA’s large, late-stage clinical trials, which primarily target amyloid, will be complete before 2025. While the lack of success in multiple amyloid trials is disappointing, it is not uninformative; AD/ADRD researchers continue to learn from each study. Moreover, NIA supports a diverse set of intervention targets (neurotransmitter receptors, cell metabolism, vasculature, growth factors, etc.); amyloid is only one of those targets. Of the more than 50 pharmacological trials supported by NIA, most investigate targets other than amyloid.
ACTC, a next-generation clinical trials infrastructure designed to harness best practices and latest methods for AD/ADRD trials, includes 35 member sites across the United States along with numerous participating sites in the United States and other countries. ACTC trials are supported by a funding opportunity for Phases Ib-III of pharmacological and non-pharmacological interventions in individuals across the AD/ADRD spectrum from presymptomatic to more severe stages of disease. A key area of focus for ACTC is improving diversity in recruitment and in the clinical trial workforce. The Minority Outreach and Recruitment Team is developing central and local partnerships with diverse communities to enhance representation of these underrepresented groups in AD/ADRD trials. The ACTC Inclusion and Diversity Committee has been conducting mentorship activities for ACTC junior investigators and trial study staff. Additionally, the ACTC Patient Advisory Board has been constituted with a focus on inclusion of individuals from underrepresented populations as well as from across the disease spectrum. Sharing of data and biosamples is another key element of the ACTC, and it is part of NIA’s enabling infrastructure for data-driven and predictive therapy development. All design, methods, procedures, etc. developed will be shared with the larger research community as will trial data and biosamples per NIA requirements noted earlier.
For more information, see:
- https://www.nia.nih.gov/news/new-nih-consortium-award-enhance-clinical-trials-alzheimers-disease-related-dementias
- https://grants.nih.gov/grants/guide/pa-files/PAR-20-309.html
In addition to ACTC infrastructure, NIH currently sponsors approximately 270 active trials of interventions to enhance cognitive health in older adults and to prevent, treat, or manage AD/ADRD. NIH also released several FOAs specifically focused on clinical trials for AD/ADRD. These include pharmacologic as well as lifestyle interventions.
For more information, see:
- https://www.nia.nih.gov/research/ongoing-AD-trials
- https://grants.nih.gov/grants/guide/pa-files/par-18-877.html
- https://grants.nih.gov/grants/guide/pa-files/PAR-18-878.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-20-029.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-22-016.html
(UPDATED) Action 1.B.6: Expand research focused on needs related to the intersection of Down syndrome and Alzheimer’s disease and related dementias
Lead Agency: NIH
In FY 2018, appropriations provided by Congress allowed NIH to not only expand its current efforts on Down syndrome and AD/ADRD, but to build an integrated effort across NIH that will be truly transformative in this area and other commonly co-occurring conditions in individuals with Down syndrome. The INvestigation of Co-occurring conditions across the Lifespan to Understand Down syndromE (INCLUDE) project was launched in June 2018 in support of a congressional directive. INCLUDE focuses on three overall goals: (1) conducting targeted, high-risk, high-reward basic science studies on chromosome 21; (2) assembling a large study population of individuals with Down syndrome; and (3) including individuals with Down syndrome in existing clinical trials. In FY 2021, Congress appropriated an additional $65 million to expand INCLUDE.
In FY 2018, NIH spent almost $23 million to jump-start INCLUDE via administrative supplements, including one focused on creating an AD/ADRD clinical trial network for adults with Down syndrome. This network, the ACTC-Down Syndrome Network aims to utilize the existing depth and breadth of expertise across its ACTC infrastructure to conduct AD/ADRD clinical trials in adults with Down syndrome. The overarching goal of the project is to build an efficient clinical trial network to address the critical need for treatment of AD/ADRD in adults with Down syndrome. The project received additional funding in FY 2020 to continue and expand this work. In FY 2019, through the INCLUDE project, a project focused on clinical trials to prevent AD/ADRD in Down syndrome was funded.
For more information, see:
- https://www.nih.gov/include-project
- https://projectreporter.nih.gov/project_info_description.cfm?aid=9749625&icde=46135063&ddparam=&ddvalue=&ddsub=&cr=1&csb=default&cs=ASC&pball
- https://projectreporter.nih.gov/project_info_description.cfm?aid=9893363&icde=46762199
Alzheimer’s Biomarker Consortium-Down Syndrome (ABC-DS) is a multi-disciplinary, multi-site longitudinal study examining biomarkers of AD in a large cohort of adults with Down syndrome ages 25 and above. ABC-DS was initiated in 2015 by NIA and National Institute of Child Health and Human Development (NICHD) with the funding of two groups of research collaborators -- Neurodegeneration in Aging Down Syndrome and Alzheimer's Disease in Down Syndrome. In September 2020, the continuation of ABC-DS was funded by NIA, NICHD and the Trans-NIH INCLUDE Project. ABC-DS researchers will follow the cohort of people with Down syndrome to conduct three projects. The next iteration of ABC-DS includes an emphasis on increasing the diversity of individuals in the cohort of adults with Down syndrome. The Alzheimer’s Disease/Down Syndrome Outreach, Recruitment, and Engagement Core will rapidly disseminate information to Down syndrome communities and engage underrepresented ethnic groups.
A recent analysis of data from ABC-DS suggests that people with Down syndrome show similar changes in metabolic processes as people with late-stage AD. The same pattern of metabolic changes occurs for people with Down syndrome who go on to develop AD and people with late-stage AD. The findings suggest that measures of metabolic substances may have potential as blood-based biomarker tests.
In 2020, NACC developed a Down syndrome-specific clinical and cognitive assessment module, implemented for use in research that is harmonized with some of the ABC-DS clinical and neuropsychological measures and available for use by ADRCs and Intellectual and Developmental Disabilities Research Centers for research purposes. Data and biosamples generated from the participants who are being evaluated with the module will also be available for broader sharing.
For more information, see:
- https://www.nia.nih.gov/research/abc-ds
- https://health.ucsd.edu/news/releases/Pages/2016-01-13-clinical-trial-alzheimers-characteristics-in-down-syndrome.aspx
- https://www.nia.nih.gov/news/people-down-syndrome-and-alzheimers-show-similar-changes-metabolic-processes-people-late-stage
- https://www.nia.nih.gov/research/blog/2021/06/teaming-expand-alzheimers-and-down-syndrome-research
- https://naccdata.org/data-collection/forms-documentation/ds-3
NIA and NICHD have also collaborated to produce and disseminate information for people with Down syndrome and their families regarding the interplay of Down syndrome and dementia, and the importance of participating in research. Efforts include a fact sheet, Alzheimer’s Disease in People with Down Syndrome, and outreach via email and social media.
For more information, see:
(ONGOING) Action 1.B.7: Issue a joint Department of Veterans Affairs/National Institute on Aging career development award for physician scientists new to the area of dementia research
Lead Agency: VA
In FY 2021, the VA funded several research proposals in response to the early career physician-scientist mentored research in AD/ADRD funding announcement. This program has been approved for another year.
(NEW) Action 1.B.8: Research the impacts of COVID-19 and Post-COVID Conditions on risk of AD/ADRD, cognition, and brain health
Lead Agencies: NIH, NIA
NIH is looking closely at the long-term effects of COVID-19 infection via the recently launched RECOVER Initiative, which is applying a meta-cohort study design to pool participants in combination with real-world data to propel multiple research studies forward, including research into potential effects on cognition and cognitive decline.
For more information, see:
- https://recovercovid.org/about
- https://www.nih.gov/news-events/news-releases/nih-builds-large-nationwide-study-population-tens-thousands-support-research-long-term-effects-covid-19
At the institute level, NIA issued its own Notices of Special Interest (NOSI) in order to stimulate much-needed research on aging and COVID-19. The NIA NOSI is intended to support administrative and revision supplements on COVID-19 related topics in the realm of neuroscience and AD/ADRD research, aging biology, behavioral and social research, and geriatrics and gerontology. In addition, NIA has issued a funding opportunity for a COVID-19 clinical trial implementation grant on aging-related topics in at-risk older adult populations, including those with cognitive impairment and AD/ADRD. In 2021, NIA also issued a NOSI to stimulate research on neurological and neurocognitive sequelae originating from SARS-CoV-2 infection in aging and age-related neurodegeneration. NINDS issued a funding opportunity titled, Impact of COVID-19 on Dementia Risk, Progression and Outcomes in AD/ADRD Populations (NOT-NS-21-037) to solicit research on the effect of COVID-19 exposure on subjects who have, or are at risk for, developing AD/ADRD.
NIA is also co-sponsoring a variety of other COVID-targeted funding opportunities, such as those specific to the Rapid Acceleration of Diagnostics Underserved Populations initiative, which seeks to enable and enhance COVID-19 testing in under-served and vulnerable populations (e.g., residents of nursing homes and assisted living facilities, individuals with cognitive impairment or dementia). More generally, NIA has provided support to its stakeholders and grantees throughout the COVID-19 Public Health Emergency (PHE), including those who work in the field of AD/ADRD. This support encompasses ongoing communications on COVID-19 related issues (e.g., multiple 2021 web updates), outreach on federal COVID-19 resources for older adults, and flexibilities for grant applicants whose research has been affected by the pandemic.
For more information, see:
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-20-022.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-20-033.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-21-016.html
- https://grants.nih.gov/grants/guide/pa-files/PAR-20-234.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-NS-21-037.html
- https://www.nia.nih.gov/research/grants-funding/nia-covid-19-response
- https://www.nih.gov/research-training/medical-research-initiatives/radx/funding#radx-up
- https://www.nia.nih.gov/research/blog/2020/03/covid-19-adjusting-new-normal
- https://www.nia.nih.gov/research/blog/2020/04/new-funding-opportunities-join-fight-against-covid-19
- https://www.nia.nih.gov/research/blog/2020/07/data-harmonization-and-sharing-are-essential-covid-19-research
- https://www.nia.nih.gov/health/government-covid-19-resources-older-adults
Strategy 1.C: Accelerate Efforts to Identify Early and Presymptomatic Stages of Alzheimer’s Disease and Related Dementias
Significant advances in the use of imaging and biomarkers in brain, blood, and spinal fluid have made it possible to detect the onset of AD/ADRD and track its progression with the hope that it will be possible to monitor the effect of treatment in people with the disease. Without these advances, these neurodegenerative processes could only be evaluated in non-living tissues. Accelerated research will improve and expand the application of biomarkers in research and practice. These advances have shown that the brain changes that lead to AD/ADRD begin up to 10 years before symptoms. Identifying imaging and other biomarkers in presymptomatic people will facilitate earlier diagnoses in clinical settings, as well as aid in the development of more efficient interventions to slow or delay progression.
(UPDATED) Action 1.C.1: Identify imaging and biomarkers to monitor disease progression
Lead Agencies: NIA, NINDS
Partners: ADNI partners, AMP partners
The Alzheimer's Disease Neuroimaging Initiative (ADNI) has contributed to much progress in neuroimaging and biomarker refinement. ADNI, a long-running, NIH-supported study, was designed to develop tools for clinical trials by tracking how neuroimaging and fluid biomarkers change with disease onset and progression. Launched by NIH in 2004, this landmark public-private partnership looks at how the evolution of clinical symptoms and neurocognitive testing in healthy controls, people with mild cognitive impairment (MCI), and people with mild AD correlates with changes in multiple biomarkers reflecting disease development. The biomarkers developed and validated in ADNI are being used more and more in clinical trials. ADNI has also pioneered rapid, transparent data-sharing while protecting participants’ privacy. Qualified researchers across the world can access ADNI brain scan images and biomarker data through a web-based portal once data are quality-controlled and added to the database. ADNI also shares the blood, cerebrospinal fluid, and DNA it has collected with other investigators who are developing novel biomarkers. Now in its 17th year, the three phases of ADNI (ADNI1/GO, ADNI2, and ADNI3) have developed biomarkers for use in selecting clinical trial participants and for assessing treatment outcomes. When ADNI3 was launched in 2016, ADNI data had already been downloaded for research purposes more than 11 million times and scientists had used ADNI data to publish more than 1,200 scientific papers.
For more information, see:
The AMP-AD program, as noted above, is an NIH led precompetitive public-private partnership to identify and validate the most promising biological targets of AD to advance diagnostic and drug development. The first phase, the AMP-AD program 1.0, consisted of two components: The Biomarkers Project and the Target Discovery and Preclinical Validation Project. The Biomarkers Project incorporated tau positron emission tomography (PET) imaging into two NIH-funded prevention trials (A4 Trial and Dominantly Inherited Alzheimer Network Trial Unit [DIAN-TU]). Data-sharing under the AMP-AD program includes making the screening data and biosamples available after enrollment completion and making post-randomization data and biosamples available as soon as possible after completion without compromising trial integrity. The Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease (A4) trial has achieved the first milestone by making the pre-screening data and biosamples available (via the Laboratory of Neuroimaging). This is the first registration trial to ever do so, and since the data was made available there have been over 600 data requests and nine publications using the A4 screening data.
For more information, see:
In 2019 and early 2020, NIH-supported scientists reported advances in the development of blood-based tests that could enable rapid screening of volunteers who wish to enroll in studies. Using a blood test to screen would reduce the number of research volunteers who undergo brain PET imaging or spinal taps, which are expensive and invasive. Since Fall 2020, physicians in clinical practice can order a blood test for amyloid protein, a hallmark sign of AD, for an individual who is not participating in a study. Several other blood tests are in development. In addition, advances were made in brain imaging, most notably the FDA-approval of the first PET scan product to detect tau tangles in the brain, another hallmark sign of Alzheimer’s. In addition to blood tests, other NIH-supported research projects are designed to look beyond current measures to identify people with dementia earlier in the disease process. These include changes in vision and pupil responses that may signal AD, or a combined decline in memory and walking speed as a sign of dementia.
For more information, see:
- https://www.nia.nih.gov/news/nia-small-business-funding-seeks-find-blood-based-diagnostic-alzheimers-disease
- https://www.nia.nih.gov/news/blood-test-shows-promise-predicting-presymptomatic-disease-progression-people-risk-familial
- https://www.nia.nih.gov/news/blood-test-method-may-predict-amyloid-deposits-brain-potentially-indicating-alzheimers-disease
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-19-012.html
To meet the pressing need to better understand the prevalence, progression, and clinical impact of Alzheimer’s among Mexican Americans, NIH awarded additional funding in 2020 for more PET scan and other biomarker measures to the ongoing Health and Aging Brain Among Latino Elders (HABLE) study. The additional funding will support the Health and Aging Brain Among Latino Elders-Amyloid, Tau, and Neurodegeneration (HABLE-AT(N)) study, which enables researchers to collect amyloid and tau PET imaging and other biomarker measures. The goal is to better understand health disparities of brain aging and AD/ADRD between Mexican Americans and non-Hispanic Whites. An additional benefit of HABLE and HABLE-AT(N) will be the ability to better classify/categorize participants into groups by type of dementia and stage of the disease. This will help facilitate potential enrollment in future studies and the tailoring of therapies as they become available.
For more information, see:
At Columbia University, investigators recruit students from underrepresented groups to conduct research projects with neuroimaging data for their NIA-funded Summer of Translational Aging Research for Undergraduates. The trainees are helping to develop brain images as biomarkers of dementia through NIA’s Advancing Diversity in Aging Research Through Undergraduate Education program.
For more information, see:
- https://grants.nih.gov/grants/guide/pa-files/PAR-17-290.html
- https://www.columbianeurology.org/education-and-training/summer-translational-aging-research-undergraduates-star-u
To enable better patient stratification, diagnosis, and tracking of disease progression in LBD, FTD, VCID, and dementias with mixed etiologies, NINDS continues to release funding opportunities to support the development of biomarkers, including imaging ligands, for AD/ADRD. For example, NINDS recently launched the Center Without Walls for PET Ligand Development for AD/ADRD. This consortium is applying cryo-electron microscopy to visualize, at atomic-level resolution, the structures of protein aggregates found in several AD/ADRD. These highly detailed structures will then be used to design and validate sensitive PET imaging probes in order to better observe differing AD/ADRD disease pathologies in patients. Such PET markers should enhance differential dementia diagnosis and serve as markers of disease progression in future AD/ADRD clinical trials.
To further our understanding of potential biomarkers for LBD, NINDS is supporting several projects and programs including AMP-PD, a public-private partnership conducting deep molecular characterization and longitudinal clinical profiling of Parkinson’s disease and LBD patient data and biosamples. In the past year, the AMP-PD program has added data and samples from patients with LBD. NIH is also continuing to support five research teams that aim to discover biomarkers that will improve the efficiency and outcome of Phase II clinical trials for LBD. In addition, NIH recently released a new funding opportunity to invite new research into the identification of biomarkers for LBD. Through this new opportunity, NIH seeks research projects that will increase our understanding of how clinical information from people with LBD corresponds with abnormal areas observed in brain tissue collected after death. In an ongoing study, NIH-funded researchers are working to develop a skin test to detect LBD-associated forms of alpha-synuclein. The team reported recently that they detected deposits of the clumped protein in skin samples from people with LBD but not in healthy people, which provides hope for the development of an easy-to-administer LBD and Parkinson’s disease biomarker.
For more information, see:
- https://grants.nih.gov/grants/guide/notice-files/NOT-NS-18-082.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-NS-21-001.html
- https://grants.nih.gov/grants/guide/pa-files/PAR-19-170.html
- https://grants.nih.gov/grants/guide/pa-files/PAS-19-210.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-16-022.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-17-016.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-19-014.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-20-012.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-20-014.html
- https://pubmed.ncbi.nlm.nih.gov/32986090/
In 2020, NINDS renewed the small vessel VCID Biomarkers Consortium (MarkVCID) program, which aims to develop and validate candidate human biomarkers for VCID that would enable more accurate identification of those at-risk for long-term cognitive decline and tracking of disease progression in individuals already affected by cognitive impairment and dementia. Several biomarker kits have been developed and preliminarily validated. These kits have now moved into Phase 2 testing for use in high thru-put clinical trials.
For more information, see:
In addition to these large initiatives, NIA and NINDS have released FOAs in the past year that call for research to further the development of imaging and biomarker research.
For more information, see:
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-18-048.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-20-017.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-NS-21-001.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-19-026.html
(UPDATED) Action 1.C.2: Maximize collaboration among federal agencies and with the private sector
Lead Agencies: NIA, NINDS
Partners: FDA, AMP partners
NIH engages in multiple partnership opportunities with the private sector and other federal agencies to facilitate collaborative efforts across the entire AD/ADRD research landscape. ADNI, the AMP-AD program, and the AMP-PD program discussed above are three large examples of these partnerships.
The NIA IMbedded Pragmatic Alzheimer’s Disease and AD-Related Dementias Clinical Trials (IMPACT) Healthcare Collaboratory received COVID-19 supplements to establish partnerships with the nursing home industry to develop data-sharing infrastructure and reporting systems to monitor the effects of the COVID-19 vaccines administered to frail older adults, on whom the vaccines were not widely tested prior to approval for use. These initiatives provided near real-time insight into the use, effects and outcomes related to use of COVID-19 vaccines among this population and electronic health record (EHR) data from nursing homes was used by the Centers for Disease Control and Prevention (CDC); the Advisory Committee on Immunization Practices) to monitor adverse events. In the near future, the nursing home EHR data will be linked with the Centers for Medicare & Medicaid Services (CMS) claims data, which can be used to improve our national response to the pandemic and public health outcomes for older adults.
For more information, see:
- http://adni.loni.usc.edu/
- https://amp-pd.org/
- https://www.nia.nih.gov/alzheimers/amp-ad
- https://impactcollaboratory.org/building-infrastructure/
Another example is the Collaboration for Alzheimer’s Prevention (CAP). CAP is a public-private partnership that brings together research groups to harmonize biomarker, clinical, and cognitive measures and align data-sharing and sample-sharing approaches used in certain trials so that findings can inform the entire research community. CAP includes researchers from three trials co-funded by NIH, industry, and foundations: the Alzheimer's Prevention Initiative, the A4 study, and the DIAN-TU. Collaborative efforts like CAP provide an effective platform for implementation of AD/ADRD research standards and advancing AD/ADRD prevention research with rigor, care, and maximal impact.
For more information, see:
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4847536/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5111162/
Also, the International Alzheimer's and Related Dementias Research Portfolio (IADRP) facilitates the tracking of research support in the public and private sectors, including the initiatives mentioned above.
For more information, see:
Strategy 1.D: Coordinate Research with International Public and Private Entities
In order to facilitate communication and collaboration, build synergy, and leverage resources, it is imperative that research across nations and across funders be coordinated. The actions below will formalize the coordination process beyond HHS and the Federal Government and make research available to the public for input.
(UPDATED) Action 1.D.1: Inventory Alzheimer’s disease and related dementias research investments
Lead Agency: NIA
IADRP, a free, searchable database providing a global overview of AD/ADRD research and funding, is an invaluable tool for assessing and planning AD/ADRD research projects. Funding organizations, researchers, and advocates are discovering IADRP's merits to help them coordinate strategies, leverage resources, avoid duplication, and identify promising areas of growth. Since NIH launched the database in 2012, in collaboration with the Alzheimer's Association, IADRP has amassed information on over 10,000 unique projects from 2008 through 2020, reflecting more than $8 billion in research funding worldwide. The number of contributors is growing, too. During the past 5 years, more than 40 funding organizations across greater than ten countries have joined the IADRP effort.
In 2018, the IADRP database was relaunched with several changes to the Common Alzheimer’s Disease Research Ontology, including greater specificity in the coding of FTD, LBD, and VCID. Additionally, users can now link research to related clinical trials, patents, and data repositories, as well as visualize search results with dynamic charts and graphs.
For more information, see:
NIH is committed to data-sharing as a way to synergize research and facilitate collaborative science while ensuring appropriate protections for research involving human data and oversight of research conduct, data quality, data management, data-sharing and data use. A collaborative approach among the major cohorts could expedite epidemiological discovery by assembling multi-level data collected across the lifespan and by providing a framework for multi-disciplinary research. NIH’s aging and AD/ADRD cohorts have been central to this mission, providing pivotal information on healthy aging and factors related to risk of and protection for AD/ADRD. A comprehensive, and publicly accessible inventory of cohorts is fundamental to facilitate collaborative scientific efforts, sharing of data and cost-effective assembly and utilization of resources. In return, this will assist the research community in the planning of new studies and will enable NIA in maximizing the returns on investments. NIA is working with the NIH Center for Information Technology to pilot-test the creation of a database for cohorts supported by NIA. The objectives of this project are to create a user-friendly cohort database of NIA’s longitudinal studies which will:
- Increase transparency and scientific quality and collaboration through public access to the aging and AD/ADRD cohort’s descriptive information.
- Assist the research community in identifying and accessing population resources for research in aging and AD/ADRD.
- Improve the return on investment in the cohorts’ infrastructure for researchers and NIA.
- Promote collaborative research projects for topics not easily addressed by a single study.
(UPDATED) Action 1.D.2: Expand international outreach to enhance collaboration
Lead Agency: NIA
NIA participated in the Alzheimer’s Disease Funders’ meeting held during the 2020 AAIC, as well as quarterly international funders’ calls led by the Alzheimer’s Association. Also, IADRP, maintained by NIA, includes data from over 40 public and private funding organizations across more than ten countries and is publicly-available for use.
For more information, see:
The NIA-supported the Health and Retirement Study (HRS) Harmonized Cognitive Assessment Protocol (HCAP) project is an innovative approach to assessing trends in cognitive function and aging in the United States and worldwide. The primary aim of the HRS, funded by NIA and the Social Security Administration, is to collect and distribute longitudinal multi-disciplinary data on a nationally representative sample of over 20,000 Americans over the age of 50 for research on aging. To provide the research community with new and richer data to study the prevalence, predictors, and outcomes of cognitive impairment and dementia, NIH first supported HCAP during the HRS’s 2016 field period. In this field period, investigators administered a supplemental in-home, 1-hour battery of cognitive tests to 3,496 randomly selected HRS respondents age 65 and older, along with a 20-minute informant interview. Many of the HCAP participants also participated in the HRS venous blood study, which is projected to yield plasma AD biomarkers when there is consensus on the best protocol for their analysis. Genotype information are already available for those HCAP (and HRS) participants who consented to being genotyped. The data from that 2016 assessment have now been made publicly-available to the scientific community and analyses are underway. A second wave of HCAP assessment was scheduled for 2020 but was postponed due to the COVID-19 pandemic. It is now projected to be back in the field as soon as the necessary home visits to the HCAP respondents are deemed to be safe. To further facilitate health disparities research, the HRS is adding 2,000 additional racial and ethnic minority respondents. By continuing to diversify this cohort, researchers using HRS data will be better able to design studies that provide insights into potential racial/ethnic differences in the incidence, prevalence, and impact of AD/ADRD.
For more information, see:
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-22-012.html
- https://hrs.isr.umich.edu/welcome-health-and-retirement-study
- https://www.src.isr.umich.edu/projects/health-and-retirement-study-harmonized-cognitive-assessment-protocol-hrs-hcap/
- https://projectreporter.nih.gov/project_info_description.cfm?aid=9618704&icde=42939202
- https://projectreporter.nih.gov/project_info_description.cfm?aid=10003934&icde=51152035&ddparam=&ddvalue=&ddsub=&cr=2&csb=default&cs=ASC&pball=
- https://projectreporter.nih.gov/project_info_description.cfm?aid=10017122&icde=51152035&ddparam=&ddvalue=&ddsub=&cr=3&csb=default&cs=ASC&pball=
- https://www.nia.nih.gov/research/blog/2019/05/healthy-cognitive-aging-project-major-data-resource-cognitive-epidemiology
- http://hrsonline.isr.umich.edu/index.php?p=shoavail&iyear=ZU&_ga=2.3082800.1242911673.1556821900-1050997585.1555960426
HCAP is also being administered in a diverse range of countries, where HRS-like representative population surveys are conducted, including China, England, India, Mexico, South Africa, Chile, and parts of the European Union. To date, the data from England, India, South Africa, and Mexico have been publicly released, and initial data release from China is expected by the end of 2021.
For more information, see:
- http://www.mhasweb.org/Data.aspx
- https://www.elsa-project.ac.uk/
- https://lasi-dad.org/
- https://haalsi.org/projects-cores
In 2019, NIA funded a research network to facilitate collaboration among longitudinal studies of aging around the world to harmonize methods and content. The ultimate goal of the HCAP Network is to develop international data resources for the study of AD/ADRD that will expand research opportunities to exploit cross-country variation in key life-course factors that likely affect cognitive function and the risk for AD/ADRD, such as educational attainment, wealth, retirement policies, diet, and the prevalence and treatment of cardiovascular risk factors. Currently, 11 studies representing countries from all over the world participate in Network activities.
For more information, see:
The rationale for NIA’s support to global AD/ADRD research and training is compelling. The wide variety of diets, health-related behaviors, and environmental exposures, as well as genetic variation within low and middle-income countries (LMICs) and their respective populations can provide valuable insight on factors that contribute or protect against AD/ADRD. Research in LMICs will not only help to mitigate AD/ADRD in the developing world but will also increase our knowledge of the complexity and heterogeneity of this disease in the global context. Data from these studies may be extrapolated to United States populations that share similar sociodemographic backgrounds to LMIC populations (e.g., race/ethnicity, low-resource, rural, etc.). To this end, NIA collaborates with the NIH’s Fogarty International Center (FIC) to support global research for AD/ADRD in LMICs. NIA is currently collaborating with FIC on the Global Environmental and Occupational Health (GEOHealth) Program with the aim to support research on environmental and occupational health threats in relation to AD/ADRD. Each NIA GEOHealth hub will be supported by two coordinated, linked awards: (1) a cooperative research award to an institution in a developing nation; and (2) a training award to a United States institution with substantial NIH involvement to coordinate research training. Over the past 19 years, NIA has also worked with FIC on the Global Brain and Nervous System Disorders Research Across the Lifespan initiative, which supports investigator-initiated and exploratory research on brain and other nervous system function and disorders throughout life in LMICs. These supported grants represent strong collaborations between United States investigators and their LMIC partners. For applications submitted in 2018-2020 NIA has supported nine grants under this initiative. NIA is working to support the development of early-stage investigators who have begun to establish research programs and who will be ready to assume leadership roles in their field of expertise and will be poised to change theory, practice, and health outcomes related to the health of older individuals in LMICs through partnership in the Emerging Global Leader Award (K43) and Institutional Training Program (D43). The Emerging Global Leader Award will provide LMIC early-stage investigators the opportunity for joint mentorship by a United States and LMIC established researcher while the Institutional Training Award will support collaborative research training between institutions in the United States and LMICs for research on chronic, non-communicable diseases including AD/ADRD.
For more information, see:
- https://grants.nih.gov/grants/guide/rfa-files/RFA-TW-21-001.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-TW-21-002.html
- https://www.nia.nih.gov/research/blog/2021/04/addressing-global-challenges-aging-and-dementia
- https://grants.nih.gov/grants/guide/pa-files/PAR-18-835.html
- https://grants.nih.gov/grants/guide/pa-files/PAR-18-836.html
- https://grants.nih.gov/grants/guide/pa-files/PAR-18-901.html
Strategy 1.E: Facilitate Translation of Findings into Medical Practice and Public Health Programs
Currently, promising research and interventions are published in the research literature and presented at scientific meetings. Additional steps are needed to highlight promising findings and to facilitate dissemination and implementation of effective interventions quickly and accurately to the general public, medical practitioners, the pharmaceutical industry, and public health systems.
(UPDATED) Action 1.E.1: Leverage public and private collaborations to facilitate dissemination, translation, and implementation of research findings
Lead Agency: NIA
Partners: FDA, ACL, CDC, partner organizations
NIA continues to expand its efforts to educate clinicians about recent research findings, clinical practice tools for assessment, diagnosis and management of cognitive impairment, training materials, and a patient checklist handout in English and Spanish, and other resources available online in a mini-portal of resources for professionals.
NIA also continues to promote research findings through press releases, research highlights, and feature articles.
In 2020, NIA and partner federal agencies led efforts to update and enhance the Alzheimers.gov website. NIA launched this new portal to Federal Government AD/ADRD information and resources in February 2021. The site features:
- Information about AD/ADRD.
- Tips and resources for caregivers and people living with dementia.
- Updates on Federal Government activities to address AD/ADRD.
- How to take part in clinical research and how to find studies.
- Resources for health care providers, community and public health workers, and researchers.
For more information, see:
- https://www.alzheimers.gov/
- https://www.nia.nih.gov/news/press-releases
- https://www.nia.nih.gov/news/featured-research
NIA is developing a web page to support the public’s understanding of cognitive assessment research and NIA-funded research in this area.
The AMP-AD Target Discovery Project has generated a wealth of molecular data from over 3,000 human brain and plasma samples collected in several NIA-supported AD cohorts and brain banks. The project makes these datasets available to the greater research community through the AMP-AD Knowledge Portal. More than 3,000 researchers around the world have used the NIH-supported AD Knowledge Portal to advance research on AD/ADRD.
In 2018, these novel target predictions, along with the data and analyses that led to their discovery, were made available via a new AMP-AD program data resource, the Agora platform. This web-based, interactive platform will enable researchers in academia, biotech and pharmaceutical communities to leverage AMP-AD program analyses and results to enhance their own work and build on the AMP-AD program discoveries.
NIH has launched the next version of the AMP-AD program (AMP-AD 2.0). AMP-AD 2.0 brings together NIH, industry, non-profit and other organizations with a shared goal of using open science practices to accelerate the discovery of new drug targets, biomarkers, and disease subtypes.
For more information, see:
- https://ampadportal.org/
- https://www.nia.nih.gov/health/alzheimers-dementia-resources-for-professionals
- https://www.nia.nih.gov/research/amp-ad
- https://www.nia.nih.gov/news/nih-invests-next-iteration-public-private-partnership-advance-precision-medicine-research
(ONGOING) Action 1.E.2: Continue to promote use of the Alzheimer’s Disease Education and Referral Center to provide evidence-based information on Alzheimer’s disease and related dementias to the public and others
Lead Agency: NIA
Partners: ADEAR, ACL, CDC, FDA, CMS, HRSA, VA, partner organizations
NIA’s ADEAR Center routinely disseminates information on AD/ADRD research findings through the NIA website, regular weekly and monthly email alerts to more than 26,000 subscribers, and social media. Progress in AD/ADRD research is also reported in the annual NIH Bypass Budget proposal.
In FY 2019, one GWEP grant recipient (University of Southern California) partnered with three Alzheimer’s Disease Centers (ADCs). The remaining 47 GWEP grant recipients that are currently funded are encouraged to work with nearby ADCs.
For more information, see:
(UPDATED) Action 1.E.3: Facilitate translation of findings into public health practice
Lead Agency: CDC
Partners: private partners
CDC provided funds to the Alzheimer’s Association through a cooperative agreement to co-develop the third in a series of Healthy Brain Initiative (HBI) Road Maps to advance cognitive health as an integral component of public health. This State and Local Public Health Partnerships to Address Dementia, the 2018-2023 Road Map project was co-authored by experts in public health and brain health, including scientists at CDC. The Road Map outlines how state and local public health agencies and their partners can continue to promote cognitive health, address cognitive impairment for people living in the community, and help meet the needs of caregivers. Twenty-five specific actions are proposed in four traditional domains of public health: educate and empower, develop policies and mobilize partnerships, assure a competent workforce, and monitor and evaluate.
Additionally, CDC has developed a series of five Issue Maps that highlight specific sets of Road Map actions related to caregiving, risk reduction, early detection, using data for action and the education of health professionals. The associated Road Map planning tool was developed to guide state and local public health professionals through quick steps in selecting Road Map actions and getting started with implementation in their jurisdictions. Two podcasts were also produced discussing the updated Road Map. In 2019, the first Road Map for Indian Country was released identifying eight priority actions for Indian Country.
For more information, see:
- https://www.cdc.gov/aging/healthybrain/roadmap.htm
- https://www.cdc.gov/aging/healthybrain/Indian-country-roadmap.html
- https://www.cdc.gov/aging/healthybrain/issue-maps/data-evidence-for-action.html
- https://www.cdc.gov/aging/healthybrain/issue-maps/early-detection.html
- https://www.cdc.gov/aging/healthybrain/issue-maps/educating-training-professionals.html
- https://www.cdc.gov/aging/healthybrain/issue-maps/risk-reduction.html
- https://www.cdc.gov/aging/healthybrain/issue-maps/supporting-caregivers.html
- https://www.cdc.gov/aging/publications/podcasts.htm
In late FY 2020, CDC made the first awards for the Building Our Largest Dementia (BOLD) Infrastructure for Alzheimer's Act to three BOLD Public Health Centers of Excellence (PHCOEs) and 16 BOLD Public Health Programs awards. Recipients to establish PHCOEs are: “Dementia Caregiving at the University of Minnesota”; “Dementia Risk Reduction at the Alzheimer’s Association”; and “Early Detection of Dementia at the New York University School of Medicine.”
States awarded at the Enhanced Level for BOLD Public Health Programs include Georgia, Minnesota, Rhode Island, Virginia, and Wisconsin. The Enhanced recipients are working on implementing their state AD/ADRD plan. At the Core Level are Colorado, Hawaii, Iowa, Maine, Mississippi, Nevada, North Carolina, Oklahoma, and Vermont, as well as Los Angeles County and the Northwest Portland Area Indian Health Board. The Core recipients are building their coalitions and starting the process of developing a jurisdictional AD/ADRD plan.
CDC awarded the second round of recipients of the BOLD Public Health Programs awards in late FY 2021. These were awarded at the Core Level to Arkansas, City of Boston, Connecticut, Idaho, Louisiana, South Carolina, and Tennessee. There are now a total of 23 public health programs who are BOLD Program recipients.
For more information, see:
- https://www.cdc.gov/aging/bold/index.html
- https://www.cdc.gov/aging/funding/phc/index.html
- https://www.cdc.gov/aging/funding/php/index.html
Additionally, CDC has partnered with The Balm in Gilead and the National Brain Health Center for African Americans (NBHCAA) to address the higher prevalence of dementias and disparities in dementia diagnosis and treatment among African Americans through strategic and culturally-appropriate public health approaches. In partnership with CDC, The Balm in Gilead garnered more than 25,000 social media impressions during the week-long “Road to Memory Sunday” campaign over the past year; had 1,000+ viewers participated in the Facebook Live Memory Sunday Town Hall, with over 300 shares including 73 Facebook Watch Parties; and distributed over 1,700 copies of The Book of Alzheimer’s and Memory Sunday Toolkit to congregations and community partners. Furthermore, they produced a four-part virtual web series to engage public health officials, health care providers, and other key stakeholders working to address today’s challenges of brain health and AD/ADRD among Black Americans and communities of color. Each session within the series aims to connect public health issues of aging with a cultural understanding of faith and spirituality. Through insightful and evidence-based discussion, participants learn how to effectively connect faith and public health approaches to raise awareness about the impacts of AD/ADRD and mobilize communities in a deliberate way to improve brain health equity.
CDC is funding and collaborating with UsAgainstAlzheimer’s (UsA2) to increase tailored messaging related to cognitive impairment, COVID-19, brain health, and AD/ADRD to Black American and Hispanic populations across the United States. In 2021, UsA2 has co-hosted three digital education events -- #SaludTues Twitter Chats -- to amplify educational content, including CDC resources, about cognitive impairment and AD/ADRD as public health issues among diverse audiences. These three events were held in partnership with the University of Texas' Salud!America arm and more than ten community-partners. The events generated 19.7 million digital impressions (impression = the total number of times the content was seen). Additionally, UsA2 has engaged partners in digital speaking events to highlight CDC resources, including a presentation on COVID-19 and Dementia at a March 16 University of Texas Dementia Caregivers Conference reaching 200 conference participants.
CDC is partnering with the National Indian Health Board (NIHB) to expand knowledge of public health practice within AI/AN communities. NIHB is expanding website content aimed at health practitioners, as well as conducting a virtual Brain Health Action Institute for Tribal Nations. This institute, facilitated by NIHB, will support tribes and Tribal organizations in using the HBI Road Map for Indian Country to start conversations, as well as develop and plan strategies for improving brain health in their own communities. The Road Map for Indian Country is the first-ever public health guide focused on dementia in AI/AN communities.
CDC contributed to the Public Health Perspectives on the Family Care Gap textbook with a book chapter that illustrates a public health approach to supporting caregivers of people with dementia using the HBI State and Local Public Health Partnerships to Address Dementia, the 2018-2023 Road Map. It is framed using essential public health services and identifies 25 strategies for public health action to support caregivers. It also addresses the anticipated family care gap and urges the collaboration of public health systems to collect data and equitably implementation of evidence-based policies and programs that support people providing care in their communities. This book was published in 2021.
CDC has updated the “Caregiving and Subjective Cognitive Decline” infographic series. The infographics were developed using 2015-2018 Behavioral Risk Factor Surveillance System (BRFSS) data from the Caregiving and Subjective Cognitive Decline (SCD) modules. The infographic populations include: national combined data, Black American, AI/AN, AAPI, Hispanic, women, men, rural, LGBT, and veterans. The state infographics for 2019 data are now available. These infographics can be used to educate the public and aid in making decisions on how to allocate resources and funding.
For more information, see:
- https://www.cdc.gov/aging/publications/briefs.htm
- https://www.cdc.gov/aging/healthybrain/brfss-faq.htm
The infographics have all been translated to Spanish.
For more information, see:
CDC updated technical assistance documents meant to provide guidance for BRFSS coordinators and researchers who would like to conduct analyses of the data collected through the BRFSS Caregiver Optional Module and the BRFSS Cognitive Decline Module. These documents provide basic computer code for analyzing the data with a goal to enable consistency in analytic methods and results reported.
Goal 2: Enhance Care Quality and Efficiency
Providing all people with AD/ADRD with the highest-quality care in the most efficient manner requires a multi-tiered approach. High-quality care requires an adequate supply of professionals with appropriate skills, ranging from direct care workers to community health and social workers to primary care providers and specialists. In order to provide culturally and linguistically appropriate services, providers should have the awareness, knowledge, and skills to work and communicate effectively in cross-cultural situations, as well as cultural humility to understand their own biases and privileges, manage power imbalances, and be open to the aspect of another person’s cultural identity that is most important to them.[16] High-quality care should be provided from the point of diagnosis onward in settings including doctor’s offices, hospitals, people’s homes, and nursing homes. Care quality should be measured accurately and coupled with quality improvement tools. Further, care should address the complex care needs that persons with AD/ADRD have due to the physical, cognitive, emotional, and behavioral symptoms of the disease and any co-occurring chronic conditions. High-quality and efficient care depends on: (1) smooth transitions between care settings; (2) coordination among health care and LTSS providers; and (3) dementia-capable health care and LTSS LTSS.
Strategy 2.A: Build a Workforce with the Skills to Provide High-Quality Care
The workforce that cares for people with AD/ADRD includes health care and LTSS providers such as: primary care physicians; specialists such as neurologists, geriatricians, and psychiatrists; registered nurses and advanced practice nurses; community health workers; social workers; psychologists; pharmacists; dentists; allied health professionals; and direct care workers, home health aides, and certified nursing assistants, who provide care across the care continuum. These providers need accurate information about furnishing care to a person with AD/ADRD including the benefits of early diagnosis, how to address the physical, cognitive, emotional, and behavioral symptoms of the disease, and how to assist caregivers as they cope with the physical and emotional aspects of their caregiving responsibilities. Enhanced specialist training is also needed to prepare these practitioners for the unique challenges faced by people with AD/ADRD. In addition, work is needed to expand the capacity of the primary care community to serve people with AD/ADRD. Dementia-specific capabilities within the direct care workforce need to be expanded and enhanced. The actions below will facilitate specific training for care professionals in order to strengthen a workforce that provides high-quality care to people living with AD/ADRD.
(UPDATED) Action 2.A.1: Educate health care providers
Lead Agencies: HRSA, VA
Partners: CMS, NIA, CDC, ACL
In FY 2020, HRSA funded 48 non-competing continuation GWEP awards. All GWEP awardees are educating and training the workforce on how to care for persons living with dementia. Of the $36.035 million GWEP budget, $8.756 million was for dementia education and training activities. In Academic Year 2019-2020 (latest available data), GWEP grants provided 906 AD/ADRD courses and training of 146,024 health care providers in AD/ADRD. In FY 2020, HRSA funded 25 non-competing continuation Geriatrics Academic Career Award (GACA) program awards totaling $1.905 million. GACA grant recipients are encouraged to provide dementia education to the workforce.
NIA produced and disseminates Assessing Cognitive Impairment in Older Patients: A Quick Guide for Primary Care Physicians and Managing Older Patients with Cognitive Impairment.
For more information, see:
- http://www.nia.nih.gov/alzheimers/publication/assessing-cognitive-impairment-older-patients
- https://www.nia.nih.gov/alzheimers/alzheimers-and-dementia-resources-professionals
In 2018 NIA released an FOA, Small Research Grant Program for the Next Generation of Clinical Researchers in AD/ADRD Research, aimed at producing trained clinical investigators pursuing careers in the field of AD/ADRD research, which is ongoing. Additionally, NIA currently supports over 80 clinical trials aimed at testing interventions to improve care for persons with dementia and their caregivers. In 2020, the NIA ACTC (described in Goal 1) launched the Institute on Methods and Protocols for Advancement of Clinical Trials in AD/ADRD course that aims to educate and promote diversity among research professionals and future researchers in the AD/ADRD field.
For more information, see:
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-19-002.html
- https://impact-ad.org/about/
- https://www.nia.nih.gov/research/ongoing-AD-trials#section5
ACL, through its Alzheimer’s Disease Programs Initiative (ADPI) program, continues to expand efforts to educate professionals (at all levels) engaged in providing care and services to persons living with AD/ADRD and their caregivers. ACL-funded programs are developing and translating tools to educate and support clinicians ranging from micro-learning modules for primary care providers and doctors to training programs tailored to community health workers. Several funded programs partner with GWEP grantees to maximize the impact that both funding streams can have in the communities they support. Since receiving approval to collect information on the training of professionals (doctors, nurses, social workers, home health aides, first responders, etc.) in 2017, ACL grantees have reported training almost 90,000 professionals through their funded state and community programs. Select training materials developed through ACL-funded AD/ADRD programs can be found on the web page of the National Alzheimer’s and Dementia Resource Center (NADRC).
For more information, see:
The VA’s Geriatric Scholars program offers staff training to integrate geriatrics into primary care practices in three training programs: (1) Intensive individual training with didactics, quality improvement coaching, and clinical practicum experiences; (2) Limited team-based training, including Rural Interdisciplinary Team Training (RITT); and (3) Self-directed learning through webinars, simulation learning, case studies, and enduring educational materials (such as dissemination of pocket cards on dementia, delirium and depression). VA Geriatric Scholars includes a wide variety of training activities, many of which include or are focused on dementia training. Examples of FY 2021 training activities included case conferences (Practical Tips for Tele-Dementia Care--Communication, Assessment, and Caregiver Education, Partnering for Dementia Care for America's Veterans, and Impact of COVID-19 on Dementia Care), a case study on Healthcare Planning and Management for Older Adults with Dementia, and simulation learning on Geriatric Patients with Cognitive Impairment. VA’s Employee Education System (EES) makes webinars available for external audiences through the TRAIN interagency sharing platform. In addition, in FY 2021, the Geriatric Scholars program piloted a Dementia Care Coordinator program for Rural Dwelling Veterans.
The Veterans Health Administration’s (VHA’s) 20 geriatric Centers of Excellence, called Geriatric Research, Education, and Clinical Centers (GRECCs), reported that their FY 2020 work included 321 dementia activities in the areas of research, education, and clinical demonstration projects. Of these GRECC dementia activities, 13% were directly related to COVID-19. Of the FY 2020 GRECC COVID-19 dementia activities, approximately 18% were research activities; 42% were education activities; and 40% were clinical demonstration project activities.
In response to the COVID-19 pandemic, CMS developed useful guidance for health care providers, health care facilities, labs, and LTSS providers on topics including telehealth, survey and certification, Medicare coverage, Medicaid coverage, and Children’s Health Insurance Program coverage, among others. For example, the Long-Term Care Nursing Homes Telehealth and Telemedicine Toolkit, a memorandum on COVID-19 Survey Activities, CARES Act Funding, Enhanced Enforcement for Infection Control deficiencies, and Quality Improvement Activities in Nursing Homes, and a compilation of COVID-19 Guidance and Updates for Nursing Homes during COVID-19.
For more information, see:
- https://www.cms.gov/files/document/covid-19-nursing-home-telehealth-toolkit.pdf
- https://www.cms.gov/files/document/qso-20-31-all.pdf
- https://www.cms.gov/files/document/covid-guidance-and-updates-nursing-homes-during-covid-19.pdf
- https://www.cms.gov/about-cms/emergency-preparedness-response-operations/current-emergencies/coronavirus-waivers
- https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
- https://www.medicaid.gov/resources-for-states/disaster-response-toolkit/coronavirus-disease-2019-covid-19/index.html
- https://data.cms.gov/stories/s/COVID-19-Nursing-Home-Data/bkwz-xpvg
(UPDATED) Action 2.A.2: Strengthen state aging, public health, and intellectual and developmental disability workforces
Lead Agency: ACL
Partner: HRSA, CDC
HHS coordinates with states to develop workforces in aging, public health, and intellectual and developmental disability (IDD) that are AD-capable and culturally and linguistically appropriate. ACL collaborated with HRSA to provide AD/ADRD training to the Aging Network.
ACL, through the NADRC, offers an annual dementia-specific webinar series that trains over 10,000 persons per year. The series targets AD/ADRD professionals, attracting family caregivers as well, and includes information on related dementias, innovative interventions, and a wide variety of caregiving topics. Continuing education units are available to attendees, and the webinars are archived on the NADRC web page. In 2019, NADRC published a new guide entitled Intellectual and Developmental Disabilities and Dementia: Practical Strategies for Professionals which provides background and strategies for professionals working with individuals living with IDD and dementia.
In 2017, ACL received Office of Management and Budget authority to collect data on professionals trained through ADPI grant funding. Since receiving approval to collect training data, ACL grantees have trained almost 90,000 professionals in the many facets of working with persons living with dementia and their caregivers.
In 2020, NADRC and ACL ADPI grantees developed a Guide to Billing Codes for Dementia Services designed to assist HCBS in the identification of sources of reimbursement for dementia services which remains an ongoing challenge. The Guide includes billing codes that select ACL grantees have used successfully to bill for dementia services. Links to additional resources are offered throughout the guide to provide guidance in using these codes as well as resources to assist in the development of the infrastructure needed to successfully bill third-party payers. In October 2020 ACL hosted a webinar to build awareness of the Guide as a tool to assist with sustainability of dementia-specific services.
NADRC annually develops tools and issue briefs on dementia-specific topics to support of paid and unpaid caregivers. New materials are disseminated through the ACL grant programs as well as at professional conferences and the NADRC website. ACL is constantly adding new tools and issue briefs to its growing library. Topics of materials developed by NADRC include, but are not limited to: advance planning, living alone, compendiums of dementia-specific interventions, and outcome measures. The NADRC website is also home to the materials delivered through the ADPI grant program. The Alzheimer’s Disease Supportive Services Programs, Alzheimer’s Disease Initiative-Specialized Supportive Services (ADI-SSS), and ADPI program deliverables determined to be potential resources for the non-grantee community are posted for review and utilization by the dementia service provider community.
For more information, see:
- https://nadrc.acl.gov/details?search1=232#result
- https://nadrc.acl.gov/details?search1=20210421105717#result
- https://nadrc.acl.gov/
Cross-agency collaborations have enhanced workforce dementia training and expertise. In FY 2020, the 48 HRSA GWEP grantees collaborated with 50 Area Agencies on Aging and seven quality improvement organizations (QIOs) to strengthen state aging, public health, and IDD workforces. In addition, 19 of the 48 GWEPs collaborated with the VA to assist with training on dementia. These collaborations persist in Year 2 of funding (FY 2020).
CDC funded the University of Illinois at Chicago (UIC) in FY 2021 as part of the HBI. Their focus is the People with IDD Healthy Brain Initiative (PwIDD-HBI) which addresses stigma, early diagnosis, and culturally and linguistically appropriate care to engage decades of collaboration with a robust network of people with IDD, their caregivers, health care providers, and community-based organizations (CBOs) that are supporting people with IDD. Guided by the HBI Road Map Series and the National Task Group on Intellectual Disabilities and Dementia Practices (NTG) “My Thinker’s Not Working” National Strategy for people with IDD, PwIDD-HBI will develop and implement public health strategies to improve the quality of life of people with IDD by raising awareness of AD/ADRD among people with IDD as a public health issue and support caregivers to care for people with IDD and care for themselves. The purpose of this project is to concentrate current efforts in the fields of AD/ADRD and IDD into one community of practice for people with IDD, caregivers (paid and unpaid), health care providers, and public health and policy stakeholders. A Healthy Brain community of practice for people with IDD will be a “one-stop space” that offers products, trainings, and materials to raise awareness of AD/ADRD among people with IDD decrease disparities for those experiencing AD/ADRD and improve people with IDD and caregiver’s quality of life.
Key accomplishments achieved include hosting the virtual HealthMatters Webinar Series, hosting five presentations supporting the HealthMatters Series which reached over 400 attendees, and authoring two publications. The HealthMatters COVID-19 2020 Webinar Series provided 19 webinars supporting brain health through the HealthMatters Program for a total of 6,463 attendees on the webinars and 1,762 YouTube views on the HealthMatters Channel. Presentations conducted by UIC and their partners include: “HealthMatters Program Virtual Coach-Health Matters Now More than Ever” (for community support providers, family members, transition to work instructors, educators, policy makers, and researchers); “Heathy Brain Initiative and Virtual Coach HealthMatters Program” (for researchers, educators, and policy makers in the Association of University Centers on Disabilities Aging Special Interest Group); Virtual Coach HealthMatters (for researchers, policy makers, practitioners, and Special Olympics Aging Task Force members); and “COVID-19 and Down Syndrome” (Down Syndrome Affiliates in Action-Board members of Down syndrome associations). Two articles were written and published with an aim in educating and empowering key stakeholders to influence health promotion and brain health policies and partnerships and to develop a competent workforce to support people with IDD in activities that promote brain health.
- Sisirak J, Janicki MP, Murphy R, Marks B, Buckley T (2020). Impact of COVID-19 on Provider Organizations Serving Adults with Intellectual and Other Disabilities. White Paper. Washington, DC: ACCESS.
- Sisirak J, Marks B, Keller S. (2021). Optimal Ageing for People with Down Syndrome. In V.P. Prasher (eds.). Excelling in Life with Down Syndrome. United Kingdom: Nova Science.
For more information, see:
(UPDATED) Action 2.A.3: Develop and disseminate a unified primary care Alzheimer’s disease and related dementias curriculum for clinical professionals and caregivers
Lead Agency: HRSA
Partners: CMS, NIA, VA
In FY 2015-FY 2017, HRSA partnered with federal staff at ACL, CDC, CMS, HHS Office on Women’s Health, and VA on a contract to develop the Dementia Curriculum for Health Care Professionals and Caregivers. The curriculum is designed to build a workforce with the skills to provide high-quality care, ensure timely and accurate detection and diagnosis, and identify high-quality dementia care guidelines and measures across care settings. The curriculum was first made available in 2017. From December 2017 to August 2020, there were 57,663 pageviews on the Alzheimer’s curriculum page. During the past 12 months, another 18,844 pageviews have been recorded. It continues to be a valuable resource for clinicians, families, and caregivers.
For more information, see:
CMS’s Resources for Integrated Care, which partners with health plans and providers, has offered a Geriatric-Competent Care Webinar Series designed to help health professionals in all settings and disciplines expand their knowledge and skills in the unique aspects of caring for older adults and in working with their caregivers, with some focus on dementia. The webinars are intended for front line community partners and delivery staff such as care managers, member service representatives, and home care providers. Topics have included: supporting people with dementia and their caregivers during the COVID-19 pandemic, empowering unpaid caregivers of older adults during times of stress and isolation, preparing the workforce to be more disability-competent, and how to use person-centered language.
For more information, see:
The VHA National Geriatrics and Extended Care Program Office, as well as its individual programs, have provided guidance and trainings to support field staff and caregivers in a variety of settings to ensure the safety of both veterans and caregivers during this unprecedented time of COVID-19 pandemic. These efforts support all VA patients, including those living with dementia. For example, guidance on limiting face-to-face visits between VA providers and veterans in Home-Based Primary Care (HBPC), Geriatric Patient Aligned Care Teams, and Medical Foster Homes (MFHs) was provided early on and has been updated to reflect the varying stages of re-opening on a regional level. Guidance for increased virtual visits was provided, which allowed multiple commercial applications to be used for communication with veterans and their caregivers. Community Adult Day Health Care (CADHC) Service Plans were amended to enable, with state approval, supportive services for veterans in their homes in lieu of attendance at CADHC Centers. Personal Protective Equipment (PPE) use recommendations for in-home care was created for veteran caregivers based on general guidance from the CDC. MFH facilitated monthly calls to provide education on creating COVID-Specific Emergency Plans, admission planning, and COVID-19 testing in Community Residential Care settings. Additionally, all programs have held regular virtual meetings with local and VA Veterans Integrated Service Network program coordinators to provide an avenue for discussion of successes and challenges as well as sharing best practices. SharePoint sites were quickly created to allow sharing of documents, online links, and other guidance to VA staff at all levels to ensure the continued safe care of our veterans.
(UPDATED) Action 2.A.4: Ensure aging and public health network providers have access to research-based up-to-date information on Alzheimer’s disease and related dementias
Lead Agency: NIA
Partners: CDC, ACL, HRSA, AHRQ, NASEM
CDC has developed a weekly newsletter that is sent out to over 67,000 subscribers, including public health professionals. The newsletters are a primary channel for disseminating information about new articles, tools, resources, and webinars related to brain health.
For more information, see:
In 2018, NIA commissioned a systematic evidence review by the Agency for Healthcare Research and Quality (AHRQ) of care and caregiving interventions for dementia in order to determine which of the interventions identified have an evidence base that is sufficient to support widespread dissemination. Findings from the AHRQ evidence review were released in 2020. In 2021, the National Academies of Science, Engineering and Medicine (NASEM) released Meeting the Challenge of Caring for Persons Living with Dementia and Their Care Partners and Caregivers: A Way Forward. The report is the culmination of a collaboration among NIA, AHRQ, and NASEM, to develop a comprehensive understanding of the evidence base for essential care and caregiving interventions for the millions of people living with dementia and their caregivers. In response to recommendations from these reports, NIA has issued new funding opportunities to support the development of rigorous, principle-based dementia care interventions that can be delivered with fidelity in a range of care settings.
For more information, see:
- https://grants.nih.gov/grants/guide/pa-files/PAR-21-307.html
- https://grants.nih.gov/grants/guide/pa-files/PAR-21-308.html
- https://effectivehealthcare.ahrq.gov/products/care-interventions-pwd/report
- https://effectivehealthcare.ahrq.gov/products/care-interventions-pwd/protocol
- https://www.nationalacademies.org/our-work/care-interventions-for-individuals-with-dementia-and-their-caregivers
- https://www.nationalacademies.org/our-work/care-interventions-for-individuals-with-dementia-and-their-caregivers---phase-two
- https://www.nap.edu/catalog/26026/meeting-the-challenge-of-caring-for-persons-living-with-dementia-and-their-care-partners-and-caregivers
NIA supports several Roybal Centers that conduct pilot research aimed at strengthening the design of dementia care interventions. In addition, the NIA IMPACT Collaboratory is designed to build the nation’s capacity to conduct pragmatic clinical trials of interventions embedded within health care systems. The IMPACT Collaboratory supports pilot studies to inform the design of larger scale pragmatic dementia care trials and demonstration projects to test, measure, and evaluate the effect of care delivery intervention programs in a health care system for people living with AD/ADRD and their care partners.
For more information, see:
- https://impactcollaboratory.org/
- https://www.nia.nih.gov/research/dbsr/edward-r-roybal-centers-translational-research-behavioral-and-social-sciences-aging
- https://www.roybalniaresearchcenters.org/
In 2020, NIA updated its list of cognitive screening tools and AD/ADRD resources for professionals and began developing a web page to illustrate NIA-funded and NIH-funded cognitive screening research and the ways that federal agencies work together in this arena. The web page is slated to launch in 2021.
For more information, see:
- https://www.nia.nih.gov/health/alzheimers-dementia-resources-for-professionals
- https://www.nia.nih.gov/health/assessing-cognitive-impairment-older-patients
In 2020, NIA, ACL, CDC, and HRSA revived and expanded the annual Focus on Aging: Federal Partners’ Webinar Series. The webinar series, which addresses important topics for public health and health care professionals, aging services organizations, the research community, and other stakeholders in aging, now features approximately three webinars per year and encompasses new federal partners. In addition to general topics of interest for older adults and those who work with them, each webinar includes information specific to individuals with AD/ADRD and their caregivers. All prior webinars are made available to the public on the Focus on Aging: Federal Partners’ Webinar Series website.
For more information, see:
- https://www.nia.nih.gov/news/focus-aging-federal-partners-webinar-series
- https://www.acpm.org/page/brainhealth
- https://effectivehealthcare.ahrq.gov/
(ONGOING) Action 2.A.5: Strengthen the ability of primary care teams in Indian Country to meet the needs of people with Alzheimer’s disease and related dementias and their caregivers
Lead agencies: IHS, CDC
Partners: VA, public and private partners
Indian Health Service (IHS) incorporated training for AD/ADRD into the online continuing education curriculum for IHS/Tribal/Urban Indian Health (UIH) program nursing. A web-based course on AD/ADRD was provided in April-June 2016 at the IHS Clinical Rounds. Results were addressed at the IHS Nursing Leadership meeting on May 17, 2016. A clinical training on diagnosis and management of AD/ADRD for an ACL/IHS/CMS LTSS conference was delivered in November 2016.
The preconference day of the 2019 National Diabetes Conference focused on geriatrics, including diagnosis and management of AD/ADRD.
There is an ongoing collaboration with the VA Greater Los Angeles GRECC and the VA Geriatric Scholars Program in team training in diagnosis and management of AD/ADRD through RITT for rural IHS and Tribal facilities and in training for support of caregivers through the IHS Addressing Challenging Behaviors in Dementia (ABCD) program.
See Action 1.E.3 for information on CDC’s partnership with NIHB to expand knowledge of public health within AI/AN communities.
(ONGOING) Action 2.A.6: Develop a baseline understanding of self-reported competence and confidence of Indian Health Service, Tribal and Urban Indian Health nursing staff in care of individuals with Alzheimer’s disease and related dementias
Lead Agency: IHS
IHS created a survey to assess nursing in IHS, Tribal, and UIH programs on self-reported competence, confidence, and recent training specific to care for individuals with AD/ADRD. The survey has been pilot-tested at one Tribal site.
(ONGOING) Action 2.A.7: Improve educational resources for primary care staff in Tribal communities caring for individuals with Alzheimer’s disease and related dementias and their families
Lead Agency: IHS
Partners: HRSA, ACL
IHS, in conjunction with HRSA, worked to pilot-test the HRSA curriculum for care of AD/ADRD in IHS, Tribal, and UIH Programs. Pending completion of the brief, targeted provider-focused curriculum, IHS will be able to report on success rates and take-up of this curriculum and further implementation in other Tribal communities.
In 2020, ACL introduced a new grant program, ADPI: Dementia Capability in Indian Country. The program is designed to bring culturally and linguistically appropriate AD/ADRD training and education and dementia-specific evidence-based interventions to Indian Country. Four tribes received 3-year awards in August 2020.
(ONGOING) Action 2.A.8: Provide decision support for clinicians in Tribal communities
Lead Agency: IHS
IHS worked to develop and pilot-test decision support tools for clinicians using the IHS EHR. As of 2018, IHS has developed templates to support the Annual Wellness Visit (AWV), including cognitive assessments and chronic care management.
(ONGOING) Action 2.A.9: Provide interdisciplinary team training in recognition, assessment, and management of Alzheimer’s disease and related dementias in small rural Indian Health Service’s facilities
Lead Agency: IHS
Partner: VA
IHS worked with the VA to provide the VA RITT to ten IHS and Tribal sites with a focus on dementia care. So far, 15 separate RITTs have been completed for more than 18 Tribal and IHS programs. The trainings include webinars, accredited through EES and TRAIN for VA and community clinicians. The latest training provided was on PTSD and Memory (January 24, 2018). Training continued in 2019 and the first quarter of 2020, and in March 2020 included the newly developed ABCD training to support public health nursing in their role of caregiver support.
For more information, see:
(ONGOING) Action 2.A.10: Understand current nursing facility staffing challenges, including the impact of COVID-19, and best practices to address them
Lead Agency: ASPE
Nursing homes require adequate staffing to provide quality care to their residents. Staffing has been shown to be an important predictor of nursing home quality. Not simply total staffing, but also the mix of professional staff and staffing stability are important factors. Despite the importance of staffing, nursing homes have historically struggled to maintain adequate staffing due to low wages, limited possibilities of advancement, and difficult working conditions. Two important drivers of these challenges have been low Medicaid rates of reimbursement and the increasing medical complexity of residents.
The COVID-19 pandemic has substantially exacerbated this staffing challenge, as nursing homes have been an epicenter of the pandemic. Already difficult working conditions have become much more difficult. Staff have been required to shoulder many new caregiving and infection control responsibilities, and they have faced hazardous working conditions, in some instances without the needed protective equipment. Staff with caregiving responsibilities also have had to deal with new challenges at home, as many daycares and schools have been closed. To help counter these challenges, both policy makers and nursing home leaders have acted quickly to attempt to help increase the supply of staff. This project will conduct analyses to understand the changes in staffing during COVID-19, factors associated with these changes, and supplement this analysis with information from key stakeholders. The Office of the Assistant Secretary for Planning and Evaluation (ASPE) has developed one report from this project, with more expected.
For more information, see:
(NEW) Action 2.A.11: Assess current capacity of dementia specialists in the United States
Lead Agency: ASPE
Partner: HRSA
The Explanatory Statement that accompanied the Consolidated Appropriations Act of 2021 directed HRSA, in consultation with ASPE, to provide a report to the Committees on Appropriations on the current capacity of the Nation's dementia specialists not later than 15 months after enactment (effective December 27, 2020). The report language says that the report should “assess provider shortages and screening capacity, identify barriers for early detection of Alzheimer's and adequate access to care, and provide recommendations to address any provider shortages and streamline the patient's Alzheimer's diagnostic pathway.”
ASPE is leading the development of the Report to Congress on the Current Capacity of Dementia Specialists. The report will include details of provider shortages and screening capacity, identify barriers for early detection of AD/ADRD and adequate access to care, and provide recommendations to both address any provider shortages and streamline the patient's AD/ADRD diagnostic pathway.
Strategy 2.B: Ensure Timely and Accurate Diagnosis
Far too many people with AD/ADRD are not diagnosed until their symptoms have become severe, particularly people of color and people of low socioeconomic status (SES). Timely diagnosis gives people with the condition and their families and caregivers time to plan and prepare for the future, leading to more positive outcomes for both. For some, the inability to access health care due to a lack of insurance or limited finances is a major concern. This is particularly important for individuals with younger-onset disease who may not yet be eligible for Medicare. Even with access to affordable care for individuals, the health care workforce needs tools that can help ensure timely and accurate diagnoses. Research has helped identify some assessment tools that can be used to detect cognitive impairment that may indicate the need for a comprehensive diagnostic evaluation for AD/ADRD. The actions below will facilitate appropriate assessment and give health care providers tools to make timely and accurate diagnoses.
(UPDATED) Action 2.B.1: Identify and disseminate appropriate assessment tools
Lead Agency: NIA
Partner: CDC
NIA-supported scientists are making important progress toward the development of highly portable, quick, versatile, and comprehensive measures of neurological and behavioral function to identify AD/ADRD at the earliest stages. Efforts include the development and validation of a combination of tests to assess MCI. The combination of tests, which are being used in the NIH-sponsored Exercise in Adults with Mild Memory Problems clinical trial of exercise, measure thinking skills such as planning, working memory, time management, and organization.
NIA also funded applications to pursue development and validation studies of cognitive screening instruments or assessments in clinical settings and to translate these screening and assessment tools into EHR systems that can assist physicians in making clinically meaningful care recommendations for patients experiencing cognitive decline.
In addition, researchers are expanding the NIH Toolbox for the Assessment of Neurological and Behavioral Functions, a dynamic set of health assessments for all ages. Now available in English and Spanish, more than 200 clinical studies are using the NIH Toolbox, and more than 250 peer-reviewed articles have been published. The NIH-supported Advancing Reliable Measurement in Alzheimer’s Disease and Cognitive Aging study is investigating the use of the NIH Toolbox measures for people with AD/ADRD. Through this effort, researchers are expanding the toolbox so that it will be valid to use with ethnically and racially diverse adults and in adults 86 and older.
For more information, see:
- https://www.nia.nih.gov/health/alzheimers-dementia-resources-for-professionals
- https://www.clinicaltrials.gov/ct2/show/NCT02814526
- https://www.healthmeasures.net/explore-measurement-systems/nih-toolbox
In 2019, NIA funded two new projects with the goal of producing smartphone applications capable of measuring cognitive status and subtle changes in cognition on mobile devices. These grants were awarded in response to a specific FOA seeking projects focused on mobile monitoring of cognitive change.
For more information, see:
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-20-017.html
- https://grants.nih.gov/grants/guide/rfa-files/rfa-ag-18-012.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-20-050.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-20-051.html
- https://reporter.nih.gov/search/BOil54h7vEyegzmJqXQYCg/projects?shared=true
- https://projectreporter.nih.gov/project_info_description.cfm?aid=9781454&icde=46515933&ddparam=&ddvalue=&ddsub=&cr=4&csb=default&cs=ASC&pball=
- https://projectreporter.nih.gov/project_info_description.cfm?aid=9781463&icde=46515933&ddparam=&ddvalue=&ddsub=&cr=9&csb=default&cs=ASC&pball=
Please see the NACC-developed Down syndrome-specific clinical and cognitive assessment module for research described in Action 1.B.6.
NINDS continues to support Consortium for Detecting Cognitive Impairment, Including Dementia (DetectCID), a national consortium to test and validate clinical paradigms that can be used in primary health care and other everyday clinical settings. The ultimate goal is to increase detection of cognitive impairment and dementia among high-risk populations, including health disparity populations, and lessen cultural and logistic barriers that currently impede both clinical care and research efforts. Three research teams across the United States are focusing on developing and validating assessment tools that are simple to use, standardized, and take 10 minutes or less to administer in a primary care setting. While investigators are still evaluating several assessment tools to determine their effectiveness and cultural appropriateness, preliminary data are encouraging. Now in Phase 2, the DetectCID research teams are scaling up their research by enrolling a larger number of research participants with at least 50% from racial and ethnic minority groups.
For more information, see:
- https://www.detectcid.org/
- https://www.ninds.nih.gov/News-Events/News-and-Press-Releases/Press-Releases/NIH-forms-new-consortium-improve-detection
- https://www.nia.nih.gov/health/assessing-cognitive-impairment-older-patients
In 2020, CDC funded the BOLD PHCOE to focus on early detection and diagnosis of AD/ADRD. The New York University PHCOE on Early Detection of Dementia brings together a broad coalition of stakeholders across the United States to assure widespread awareness of why early detection of dementia matters. The Center created a comprehensive three-part strategy to ensure a thorough scoping review of materials for early detection and diagnosis of AD/ADRD including: (1) collate public facing educational materials from websites including those of CDC, Alzheimer’s Association, and NIA; (2) conduct a series of web-based searches for educational materials, tools, checklists, protocols, and resources designed to facilitate detection and early diagnosis of dementia; and (3) search the peer-reviewed literature using primarily PubMed to identify articles providing evidence of successful tools and strategies for early detection. As of April 2021, the Center has initiated this first strategy by gathering 13 pages of weblinks to resources with descriptions. The goal is to publish all educational materials and peer-reviewed literature on the evidence of successful tools and strategies for early detection and diagnosis of AD/ADRD on their website for public access and use.
(UPDATED) Action 2.B.2: Educate family and service providers of persons with intellectual and developmental disability about changes that may indicate the onset of dementia
Lead Agency: ACL/AIDD
Partner: NTG
ACL is providing dementia-capability training to paid and unpaid caregivers of persons living with dementia, including individuals living with IDD and dementia or at risk of developing dementia, through grants to states and CBOs. For example, over 8,000 paid and unpaid caregivers were trained on IDD and dementia through ACL-funded state and community programs. ACL collaborates closely with the NTG with many grantees participating in their education workshops, becoming affiliated trainers and further disseminating education on IDD and dementia, as well as implementation of their Early Detection Screen for Dementia tool.
ACL consistently offers educational opportunities and resources in support of both paid and unpaid caregivers of those living with IDD and AD/ADRD or at risk of developing AD/ADRD. IDD and dementia are consistently included in ACL’s annual webinar series. Webinars have focused on important topics including early screening, palliative care, family advocacy, and promising practices in care. In October 2020 ACL funded, through the NADRC, a recorded training entitled Adapting Evidence-Based and Informed Caregiver Interventions to Support Caregivers of People with Intellectual and Developmental Disabilities and Dementia to train people to support caregivers of people living with IDD and dementia.
For more information, see:
CDC, through its HBI, is funding efforts to tailor dementia public health messaging and resources towards persons with IDD. See Action 2.A.3 for information on CDC’s funding of the PwIDD-HBI.
For more information, see:
(UPDATED) Action 2.B.3: Increase awareness of Alzheimer’s disease and related dementias in Tribal and Urban Indian communities and of the availability of services for individuals with dementia and their families
Lead Agencies: IHS, CDC, NIA
Partners: ACL, VA
IHS, with ACL and VA, pilot-tested AD/ADRD awareness strategies in communities in which Resources for Enhancing Alzheimer's Caregivers Health (REACH) into Indian Country was successfully implemented through both health care and aging services settings. The focus of the REACH intervention in its final year was on increasing awareness of AD/ADRD in those communities served by REACH and increasing use of REACH caregiver support services. IHS facilities provide local resources for community-based education and training.
ACL continues to bring awareness to AD/ADRD in Indian Country through participation in educational opportunities including presentations to attendees of ACL’s Title VI annual conference and webinars. In 2020, ACL introduced a new ADPI grant program, Dementia Capability in Indian Country. In August 2020, four Tribal entities were funded to develop and implement culturally and linguistically appropriate education programs and deliver evidence-based interventions in support of elders living with dementia and their caregivers.
CDC, in partnership with National Council of Urban Indian Health (NCUIH), developed a communication campaign to raise awareness of AD/ADRD for urban Indian Elders. The campaign includes videos, a social media toolkit, flyer and posters, graphics, and other resources.
For more information, see:
A number of NIA’s ADRCs are working in Native Communities and have developed brochures and videos that encourage the participation of AI/AN in AD/ADRD clinical trials so that the prevalence of the disease in Native communities can be better understood. Additional resources include information about AD/ADRD, caregiver resources, and research education.
For more information, see:
(UPDATED) Action 2.B.4: Increase provider awareness of the need for early diagnosis, and provide tools and resources to enable diagnosis and referral
Lead Agency: CDC
Partner: private partners
CDC funds a special interest project at the University of Washington School of Medicine through CDC’s Prevention Research Centers that aims to improve cognitive impairment detection and referral to resources among older adults by applying the Gerontological Society of America’s KAER Model to primary care within a health care system. The project will increase awareness of early signs, detection of cognitive impairment, and support of providers, patients, and caregivers to ultimately improve outcomes for care of dementia. The project will implement an education intervention for primary care providers and clinical staff to increase skills for evaluation and management of dementia. Working with the University clinic managers and information technology, the project will streamline operations and document care utilizing newly developed interdisciplinary workflows and EHR order sets. A Community Advisory Board will assist in the development of a web-based resource directory to be used in-clinic and at home to support providers, staff, patients, families, and caregivers. It is anticipated that by the end of the 2-year project, strategies developed and implemented will help other health care systems initiate steps to integrate the KAER model and other tools for improving detection and management of dementia through support of primary care.
For more information, see:
- https://www.acpm.org/page/brainhealth
- https://www.cdc.gov/aging/bold/index.html
- https://www.cdc.gov/aging/healthybrain/issue-maps/early-detection.html
- https://www.geron.org/images/gsa/kaer/gsa-kaer-toolkit.pdf
Also see Action 2.A.5 for an update on the work of the CDC with support from the BOLD Infrastructure for Alzheimer’s Act to create a uniform national public health infrastructure.
Strategy 2.C: Educate and Support People with Alzheimer’s Disease and Related Dementias and Their Families upon Diagnosis
Sometimes, even though a physician or another health care provider has identified cognitive impairment, the patient and his/her family and caregivers are not told of the diagnosis. Further, once a diagnosis is made and disclosed, as few as half of patients and families receive counseling, support, or information about next steps. This information is important, especially for early-stage individuals who may experience positive outcomes when they are involved in planning and receive appropriate services. The actions below will address this gap by educating physicians and other health care providers, incentivizing discussions with people with AD/ADRD and their families and caregivers, and enhancing the ability of other networks to assist people living with AD/ADRD and their families to address their needs.
(ONGOING) Action 2.C.1: Educate physicians and other health care providers about accessing long-term services and supports
Lead Agency: HRSA
Partners: CMS, ACL
One barrier to counseling and support is that health care providers are not aware of available services or how to access them. To increase knowledge of these resources among physicians, nurses, and hospitals, HRSA grantees are working with federal partners, public and private entities, the health care provider community, and community organizations that provide LTSS to effectively educate physicians and other health care providers, direct services workers, and patients, families, and caregivers about support resources and services available to assist people with AD/ADRD, as well as their caregivers. These activities will continue as part of the training in Action 2.A.1.
CMS makes a separate Medicare payment for cognitive assessment and care planning services for individuals with cognitive impairment. A required element of these services is the creation of a care plan, including required referral to community resources as-needed (e.g., rehabilitation services, adult day programs, support groups), and that the care plan is shared with the patient and/or caregiver with initial education and support. The 2022 Medicare & You booklet highlighted this important service for beneficiaries and caregivers, and CMS also created a related video for providers.
For more information, see:
- https://www.medicare.gov/Pubs/pdf/10050-medicare-and-you.pdf
- https://www.youtube.com/watch?v=NmDjhRVax8E
ACL’s state and community ADPI program continues to fund projects that include significant focus on the provision of educational opportunities for physicians and other health care providers. Grantees continue to work toward developing models such as dementia-capable hospitals and federally qualified health centers, including educating providers on the importance of dementia-capable care transitions.
(UPDATED) Action 2.C.2: Connect American Indians and Alaska Natives to Alzheimer’s disease and related dementias resources
Lead Agency: IHS
Partners: ACL, CDC
The focus on increasing support to caregivers in Tribal Communities has been through the spread of REACH into Indian Country, with the goal of offering this intervention to those with AD/ADRD and their families. IHS collaborated with the CDC and Alzheimer’s Association to develop the Road Map for Indian Country designed specifically for Tribal Communities. The Road Map was released and disseminated to multiple stakeholders and Tribal Nations. Additionally, CDC developed an infographic describing SCD and related functional limitations, as well as caregiving, in AI/AN adults in order to educate stakeholders and policy makers on brain health in Indian Country. The International Association for Indigenous Aging (IA2), a recipient of HBI support, and the Northwest Portland Area Indian Health Board are developing, tailoring, and disseminating AD/ADRD materials and resources to AI/AN communities.
Tribal communities continue to benefit from ACL’s state and community AD/ADRD grant programs, including development of culturally-competent dementia care specialists, dementia-friendly community education/awareness initiatives, and translation of the Music and Memory intervention in Indian Country. ACL’s Title VI program has significantly increased the AD/ADRD educational offerings at their annual Title VI Training Conference including increasing awareness of CDC’s Road Map for Indian Country. In 2020, a new ADPI grant program was introduced, Dementia Capability in Indian Country. In August 2020, four Tribal entities were funded to develop and implement culturally and linguistically appropriate education programs and deliver evidence-based interventions in support of elders living with dementia and their caregivers. The recipients of the ACL Dementia Capability in Indian Country grants included Tribal Senior Services, a Tribal Health System, an Inter-Tribal Council and a regional association that serves a broad range of Tribal needs.
For more information, see:
- https://www.cdc.gov/aging/healthybrain/Indian-Country-resources.html
- https://www.cdc.gov/aging/healthybrain/Indian-country-roadmap.html
CDC has partnered with the NCUIH to review, adapt, and disseminate existing culturally and linguistically appropriate flyers and posters on brain health for an urban AI/AN audience. To accompany the updated materials, NCUIH, with support and feedback from NCUIH staff, created three culturally-appropriate one-minute videos on:
- Preventing Alzheimer’s.
- Recognizing the signs of Alzheimer’s in loved ones.
- Healthy living with Alzheimer’s.
To ensure maximum exposure, NCUIH will develop and disseminate a healthy brain media kit for use by UIHs and others wanting to raise awareness on AD/ADRD and healthy aging. The media kit will include the updated flyers and posters and links to the videos and social media campaign messaging information. Finally, NCUIH will launch a social media campaign with targeted outreach in cities with the largest AI/AN population (Los Angeles, Phoenix, Tulsa, Oklahoma City, and Anchorage) to disseminate all materials.
Additionally, CDC developed an infographic describing SCD and related functional limitations and caregiving on AI/AN adults in order to educate stakeholders and policy makers on brain health in Indian Country. These are available in both English and Spanish.
For more information, see:
- https://www.cdc.gov/aging/healthybrain/videos/index.html
- https://www.cdc.gov/aging/data/infographic/2018/american-Indian-alaska-native-cognitive-decline.html
- https://www.cdc.gov/aging/spanish/infographic/2018/aian-cognitive-decline.html
- https://www.cdc.gov/aging/data/infographic/2018/american-indian-adults-caregiving.html
- https://www.cdc.gov/aging/spanish/infographic/2018/ai-adults-caregiving.html
Also see Action 1.E.3 for information on CDC’s partnership with NIHB to expand knowledge of public health within AI/AN communities.
Strategy 2.D: Identify High-Quality Dementia Care Guidelines and Measures Across Care Settings
Guidelines for the delivery of high-quality care and measures of quality care are needed to ensure that people with AD/ADRD receive high-quality, culturally and linguistically appropriate care in the many different settings where they receive services. These guidelines and measures should be tailored to the stages of the disease, address the physical, cognitive, emotional, and behavioral symptoms of AD/ADRD, and cover the myriad of care settings in which care is delivered. These guidelines should also take into account how care might be modified for diverse populations and in the context of co-occurring chronic conditions in people with AD/ADRD. HHS will seek expert input from public and private entities, and ensure that content builds on existing, evidence-based guidelines. Quality measures should be based on such guidelines and track whether recommended care is being provided. Guidelines and measures need to be free of conflicts of interest. The actions below will advance the development of guidelines and measures of high-quality care, as well as the ability of the provider community to improve the quality of the care they provide. In the future, to facilitate the implementation of quality care guidelines and measurement, HHS will explore development and electronic sharing of clinical decision support interventions in concert with guidelines and measures to provide physicians the information they need at the point of care and ensure continuity between measurement, evaluation, and best practice.
(ONGOING) Action 2.D.1: Explore dementia care guidelines and measures
Lead Agency: CMS
Partner: ASPE
CMS has included dementia-related measures in the Merit-based Incentives Payment System for Medicare such as cognitive assessment, education and support of caregivers, and other measures that impact people with dementia. In late 2020, CMS published a Request for Information to solicit information from the public on a “core set” of HCBS measures that could impact Medicaid beneficiaries enrolled in HCBS programs, which includes people with dementia.
For more information, see:
- https://qpp.cms.gov/mips/quality-measures
- https://www.medicaid.gov/medicaid/quality-of-care/improvement-initiatives/hcbs/index.html
- https://www.cms.gov/newsroom/press-releases/cms-seeks-feedback-performance-medicaid-funded-home-and-community-based-services
(ONGOING) Action 2.D.2: Solicit stakeholder input on meaningful outcomes to drive quality measurement
Lead Agency: CMS
Partners: ASPE
CMS’s “Meaningful Measures” framework identifies the highest priorities for quality measurement and improvement. It involves only assessing those core issues that are the most critical to providing high-quality care and improving individual outcomes. The Meaningful Measure Areas serve as the connectors between CMS’s goals and individual measures and initiatives that demonstrate how high-quality outcomes are being achieved across settings of care.
For more information, see:
(ONGOING) Action 2.D.3: Clarify and disseminate information on privacy, autonomy, and safety issues to physicians
Lead Agency: HRSA
HRSA worked to develop information for physicians on privacy, autonomy, and safety issues around AD/ADRD. These resources are intended to help providers better understand these issues and the balance between safety, privacy, and autonomy. HRSA continues to disseminate this information through the trainings provided by the GWEP awardees.
(ONGOING) Action 2.D.4: Provide improved training resources to Indian Health Service staff on person-centered goals and strategies for care improvement
Lead Agency: IHS
Partners: HRSA
IHS collaborated with HRSA to engage the HRSA-funded GWEPs on strategies to improve recognition and diagnosis of dementia. Eleven HRSA GWEPs are currently partnering with federally recognized Tribal organizations. The 11 GWEPs participate in a Native Populations Interest Group for the purpose of exchanging training materials and collaborating regionally on providing education and training to native peoples. The University of Wyoming, in partnership with members of the Eastern Shoshone and Northern Arapaho tribes, completed a culturally-relevant dementia training material for American Indian people on the Wind River Reservation by creating a pictorial version of the Alzheimer’s Association’s “Know the 10 Signs: Early Detection Matters”. This continues to be disseminated as a national resource.
(ONGOING) Action 2.D.5: Improve nursing home care and transparency during the COVID-19 pandemic
Lead Agencies: CMS, VA
CMS announced a COVID-19 transparency effort to keep nursing home residents safer during the PHE by informing residents, their families, and representatives of COVID-19 cases in their facilities. Nursing homes must publicly report cases of COVID-19 to the CDC. CMS publishes this information to the COVID-19 Nursing Home Data website so the public can view information on the cases and deaths among nursing home residents and staff in the roughly 15,000 nursing homes reporting this data. CMS also published updated guidance about flexible visitation policies in nursing homes to help combat the impact of the pandemic on social isolation and mental health of residents and their families. CMS has also published ongoing guidance and hosted calls for partners, beneficiaries and other stakeholders on pandemic-related information.
For more information, see:
- https://data.cms.gov/stories/s/COVID-19-Nursing-Home-Data/bkwz-xpvg/
- https://www.cms.gov/files/document/qso-20-39-nh.pdf
- https://www.cms.gov/outreach-education/partner-resources/coronavirus-covid-19-partner-resources
- https://www.cms.gov/files/document/qso-20-39-nh-revised.pdf
CMS tasked a contractor to convene the independent Coronavirus Commission on Safety and Quality in Nursing Homes to comprehensively assess the nursing home response to the COVID-19 PHE. The Commission included representatives from states’ Departments on Aging, various health care systems, and multiple provider associations and released a report in 2020 that identified 27 recommendations and accompanying action steps to improve care. Some of these recommendations include updating cohorting guidance to better address mental health needs of both staff and residents, providing workforce hazard pay to staff, and improving upon health information technology (HIT) to better integrate nursing home data with that of other health care systems.
For more information, see:
In order to ensure the residents in the 134 VA Community Living Centers (CLCs, formerly known as VA Nursing Home Care Units) were protected, admissions were curtailed, and staff were limited to only those essential to the care of the veterans. VA CLCs continued the practice of consistent staff assignment to help with minimizing the potential spread of COVID-19 and to promote this best practice that supports individuals with dementia as well as other residents. To protect the residents by reducing the probability of COVID-19 entering the CLCs, outpatient visits were cancelled unless medically necessary and appointments were facilitated using iPads. The VA Office of Connected Care provided additional iPads to connect CLC residents with family and friends. VA CLCs’ QIO, called Community Living Centers’ Ongoing National Center for Enhancing Resources and Training, helps CLCs share innovative practices for increasing meaningful engagement and reducing the impact of social isolation, which are particularly difficult for residents with dementia and others during this pandemic. VA CLCs are also participating in a 1-year COVID-19 research study begun in 2020 and funded by NIA. The overall study objective is to describe the differential impact of COVID-19 on nursing home residents with AD/ADRD versus those without AD/ADRD, on clinical presentation and COVID-19 testing, outcomes and spread.
(NEW, COMPLETED) Action 2.D.6: Study the impacts of COVID-19 on foregone care of older adults, including those with Alzheimer’s disease and related dementias
Lead Agency: CMS
CMS published survey information about Medicare beneficiaries’ experience during the COVID-19 pandemic. The most common types of foregone care were dental care, followed by regular check-up, treatment for ongoing condition(s), and diagnostic or medical screening test. Respondents stated care was foregone due to not wanting to be at-risk in a medical facility. Beneficiaries also reported feeling more stressed and anxious, lonely or sad, less financially secure, and less socially connected to family and friends.
For more information see:
Strategy 2.E: Explore the Effectiveness of New Models of Care for People with Alzheimer’s Disease and Related Dementias
Work is underway at a number of agencies to identify models that provide more effective and efficient care for people with AD/ADRD. Models that improve health and quality of life for people eligible for both Medicaid and Medicare (dually eligible) are also of great importance to the AD/ADRD population, as approximately 19% of the dually eligible population has some form of dementia.
(ONGOING) Action 2.E.1: Evaluate the effectiveness of relevant Innovation Center models for people with Alzheimer’s disease and related dementias
Lead Agency: CMMI
Partners: NIA, Johns Hopkins University
CMS’s Innovation Center has tested several initiatives payment and service delivery models, including models aimed at improving care for Medicare beneficiaries with AD/ADRD. Several awards under the Innovation Center’s Health Care Innovation Awards rounds 1 and 2 were focused on people with AD/ADRD and their caregivers.
For more information, see:
- https://innovation.cms.gov/initiatives/Health-Care-Innovation-Awards/
- https://innovation.cms.gov/innovation-models/health-care-innovation-awards/round-2
(ONGOING) Action 2.E.2: Evaluate the effectiveness of the Independence at Home Demonstration
Lead Agency: CMMI
The Independence at Home Demonstration is testing a payment incentive and service delivery model that uses physicians and nurse practitioners to coordinate HBPC with HCBS. CMS has released the results from Year 5 of the demonstration.
For more information, see:
- https://innovation.cms.gov/files/fact-sheet/iah-yr5-fs.pdf
- https://innovation.cms.gov/initiatives/independence-at-home/
(UPDATED) Action 2.E.3: Understand the role of certified community behavioral health clinics in providing access to care
Lead Agency: ASPE
In April 2014, the Protecting Access to Medicare Act created the Certified Community Behavioral Health Clinic (CCBHC) demonstration. The demonstration establishes a standard definition for CCBHCs and allows states to develop new prospective payment systems (PPS) that reimburse CCBHCs for the total cost of providing comprehensive services to all individuals who seek care. ASPE is managing a 5-year evaluation of this demonstration to answer research questions on how the CCBHCs improve access to care, whether they implement a full scope of services, how they improve the quality of care, whether the PPS covers the full costs of care, and how the demonstration impacts costs and utilization in Medicaid. Reports to Congress are due annually, and both interim reports and a final report will be posted on ASPE’s website. The first Report to Congress was published in 2017; three additional Reports to Congress have been transmitted, and two interim reports were published in September 2020. The final Report to Congress will be transmitted to Congress in 2021, and the final report will be posted on ASPE’s website. Plans are underway to evaluate the demonstration which has been extended and expanded.
For more information, see:
- https://www.samhsa.gov/sites/default/files/ccbh_clinicdemonstrationprogram_071118.pdf
- https://aspe.hhs.gov/report/certified-community-behavioral-health-clinics-demonstration-program-report-congress-2018
- https://aspe.hhs.gov/basic-report/certified-community-behavioral-health-clinics-demonstration-program-report-congress-2019
- https://aspe.hhs.gov/basic-report/preliminary-cost-and-quality-findings-national-evaluation-certified-community-behavioral-health-clinic-demonstration
- https://aspe.hhs.gov/report/implementation-findings-national-evaluation-certified-community-behavioral-health-clinic-demonstration
Strategy 2.F: Ensure that People with Alzheimer’s Disease and Related Dementias Experience Safe and Effective Transitions between Care Settings and Systems
People with AD/ADRD have higher rates of emergency department visits and hospitalizations, two settings where they are vulnerable to stress, delirium, and unnecessary complications. A transition between providers and care settings is a complex time of care delivery for all people, but especially for frail older adults and people with AD/ADRD, who often have multiple chronic conditions. Transitions include moves into acute care hospitals, from hospitals to post-acute care (PAC) settings, such as skilled nursing facilities, or the home, or from nursing facilities to hospitals. People with AD/ADRD are at high risk of adverse events due to poor communication and other care process deficiencies during transitions and need support to help them determine the best timing for transition and site of care.
(ONGOING) Action 2.F.1: Implement and evaluate new care models to support effective care transitions for people with Alzheimer’s disease and related dementias
Lead Agency: CMS
Partner: ACL
CMS’s Primary Care First model is a voluntary Innovation Center payment model that rewards value and quality by offering an innovative payment model structure to support the delivery of advanced primary care.
For more information, see:
ACL’s state dementia system grants continue to require a care transitions component and an evaluation of the effectiveness of these programs. Numerous innovative evidence-based and evidence-informed models of care transitions interventions are presently being implemented through ACL’s state projects. Information on promising program practices is disseminated through the NADRC.
Through the ADPI state and community grants program, the ACL funds the piloting of innovations in care transitions programs. In Nevada, ACL has funded a successful Hospital2Home intervention to deliver dementia-capable supports as persons living with dementia being discharged from the hospital.
For more information, see:
(COMPLETED) Action 2.F.2: Understand facility-initiated involuntary discharges from nursing homes
Lead Agency: ASPE
Prior to the COVID-19 pandemic, media news and Ombudsman programs reported that facility-initiated involuntary discharges were becoming a leading cause of complaints for nursing home residents. As a proxy for facility-initiated discharges, this study identifies resident characteristics related to increased risk of live discharges, which included low income residents transitioning to Medicaid eligibility. Among other things, ASPE found that transition to Medicaid eligibility over a 3-6 months prior to discharge was more prevalent among a resident discharged live than those not discharged. Residents with certain risk factors, such as severe behavioral symptoms, impairments requiring more staff time, transitioning to Medicaid, and with psychiatric and mood disorders were at a higher risk of being discharged and more likely to be discharged from for-profit, government and chain facilities than non-profit and non-chain facilities. There were high rates of acute care use and mortality among residents discharged live from nursing facilities. Rates of post-discharge acute care use were higher among residents with risk factors than residents without risk factors. A report on these findings will be posted to the ASPE website in 2022.
(ONGOING) Action 2.F.3: National Center on Advancing Person-Centered Practices and Systems
Lead Agency: CMS
Partner: ACL
National Center on Advancing Person-Centered Practices and Systems (NCAPPS), an initiative between CMS and ACL to implement person-centered practices, issued a tool, the Health Care Person-Centered Profile. The template includes essential health information and a format for outlining what is important to the person who may be hospitalized and unable to communicate their wants, needs, and preferences. NCAPPS has other resources on direct support professional recruitment and retention, person-centered planning facilitation, and inclusion.
For more information, see:
(UPDATED) Action 2.F.4: Release Guide to Billing Codes for Dementia Services
Lead Agency: ACL
In September 2020, ACL released, through the NADRC, a new Guide to Billing Codes for Dementia Services. Dementia services and supports play an important role in helping people who are living with dementia to remain in the community. Identifying sources of reimbursement for dementia services remains an ongoing challenge. This Guide is intended primarily for organizations that have medical billing systems in place and want to understand how to bill for certain dementia services. It may also be useful for organizations that are considering developing a medical billing system for services. In October 2020, the NADRC hosted a webinar entitled Sustaining Programs for People Living with Dementia and their Caregivers: Billing for Dementia Services.
For more information, see:
- https://nadrc.acl.gov/details?search1=232#result
- https://nadrc.acl.gov/details?search1=20210421105717#result
Strategy 2.G: Advance Coordinated and Integrated Health and Long-Term Services and Supports for People Living with Alzheimer’s Disease and Related Dementias
Coordinating the care received by people with AD/ADRD in different settings by different providers can help reduce duplication and errors and improve outcomes. Despite a general consensus that care coordination is important, more research is needed to determine how best to provide such care in a high-quality and cost-efficient manner. The actions under this strategy will focus on learning from the existing evidence regarding care coordination and using this information to implement and evaluate care coordination models for people with AD/ADRD.
(ONGOING) Action 2.G.1: Implement and evaluate care coordination models
Lead Agency: CMS
CMS makes payment for care management and coordination services, including complex and transitional care management. Care coordination models can be a critical component of care in Medicare that can contribute to better health outcomes and higher beneficiary satisfaction.
For more information, see:
- https://www.cms.gov/About-CMS/Agency-Information/OMH/equity-initiatives/chronic-care-management.html
- https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/ChronicCareManagement.pdf
(ONGOING) Action 2.G.2: Assess the adequacy of health information technology standards to support the needs of persons with Alzheimer’s disease and related dementias
Lead Agency: ONC
Partners: CMS, ASPE
HIT is an essential tool to facilitate enhanced care coordination and communication between health care and human service providers that support patients with AD/ADRD. The Office of the National Coordinator for Health Information Technology (ONC) publishes an annual Interoperability Standards Advisory to bring public awareness to inter-operability standards and implementation specifications that can be used by industry, including standards that support care plans and transitions in care, among others.
In September 2019, the standards development organization, Health Level 7, published the eLTSS Fast Healthcare Interoperability Resource standards which are now available for adoption and implementation by HIT vendors, including those that develop dementia care technology platforms.
For more information, see:
(COMPLETED) Action 2.G.3: Study the impacts of managed care on health outcomes and quality
Lead Agency: ASPE
ASPE completed a project, Aligning Medicaid and Medicare Advantage Managed Care Plans for Dual-Eligible Individuals, that evaluated the impact of Medicare and Medicaid alignment for individuals eligible for both Medicare and Medicaid on beneficiary outcomes and utilization of acute care services and LTSS. Nineteen percent of all Medicare-Medicaid dually eligible beneficiaries have AD/ADRD. The project focused on one state (Tennessee) that implemented Medicare and Medicaid alignment for dually eligible beneficiaries through a combination of Medicare Advantage Dual Eligible Special Needs Plans contracting and Medicaid managed long-term services and supports. In Tennessee, increased aligned plan participation was associated with small decreases in nursing home use and increases in HCBS use among older adults, which is consistent with Tennessee’s goal of rebalancing LTSS towards more home and community-based settings. Some data indicates that inpatient and emergency department use initially increased for dually eligible beneficiaries under age 65 as participation in aligned plans increased, but these associations weakened or were no longer present when the analysis included more years of data. The final report was published in the journal Medical Care Research and Review. It was also featured on the Better Care Playbook, allowing for increased public dissemination.
For more information see:
- https://pubmed.ncbi.nlm.nih.gov/34075825/
- https://www.bettercareplaybook.org/resources/aligning-medicaid-and-medicare-advantage-managed-care-plans-dual-eligible-beneficiaries
Strategy 2.H: Improve Care for Populations Disproportionately Affected by Alzheimer’s Disease and Related Dementias and for Populations Facing Care Challenges
Some populations are unequally affected by AD/ADRD, including racial and ethnic minorities and people with IDD. Most racial and ethnic minority groups are at greater risk for developing AD/ADRD and face barriers to obtaining a diagnosis and services after onset. People with Down syndrome almost always develop AD/ADRD as they age. In addition, because AD/ADRD primarily affects older adults, the population with younger-onset AD/ADRD faces unique challenges with diagnosis, care, and stigma. HHS will undertake the actions below to better understand the unique challenges faced by these groups and create a plan for improving the care that they receive, which will be integrated into the broader efforts to improve care for all people with AD/ADRD.
(UPDATED) Action 2.H.1: Create funding opportunities for organizations to improve care for these specific populations
Lead Agency: ACL
In 2020, 16 AD/ADRD community-based projects, including four Tribal communities, were funded through ACL’s ADI-SSS program. As of August 2021, an additional 11 state and community programs and one Tribal program were funded, bringing the program total to 117 state, community, and Tribal programs funded in the United States and its territories since 2014. Profiles of ACL-funded projects are available for viewing on the NADRC website. Future grants through ADPI are contingent on availability of funding.
For more information:
- https://nadrc.acl.gov/details?search1=20210602113141#result
- https://acl.gov/news-and-events/announcements/alzheimers-disease-programs-initiative-adpi-dementia-capability-0
- https://nadrc.acl.gov/
(UPDATED) Action 2.H.2: Target resources towards the intellectual and developmental disability and dementia population
Lead Agency: ACL
Through its AD/ADRD grant programs and NADRC, ACL continues to target program resources to addressing IDD and dementia. For example, the NADRC includes an IDD and dementia-specific webinar in their annual webinar series, which can be found on the NADRC website.
Many ACL-funded programs use their resources to develop tools designed to support people living with IDD and dementia. Tools that demonstrate positive impact on the intended audience are made available to the public through the NADRC website.
In 2019, the NADRC published a new guide entitled Intellectual and Developmental Disabilities and Dementia: Practical Strategies for Professionals which provides background and strategies for professionals working with individuals living with IDD and dementia.
Through the NADRC, ACL-funded a recorded training entitled Adapting Evidence-Based and Informed Caregiver Interventions to Support Caregivers of People with Intellectual and Developmental Disabilities and Dementia in October 2020. The training educated viewers on ways to support caregivers of people living with IDD and dementia.
For more information, see:
- https://nadrc.acl.gov/details?search1=20210225025851#result
- https://nadrc.acl.gov/details?search1=169#result
- https://nadrc.acl.gov/
Also see Action 2.A.3 for more information on CDC’s funding of the PwIDD-HBI.
(ONGOING) Action 2.H.3: Adapt care provision strategies for Tribal members during the COVID-19 pandemic
Lead Agency: IHS
IHS’s Tribal, and UIH programs rapidly shifted care from in-person to telephone and video-based visits to limit risk of exposing elders with dementia and other at-risk individuals to COVID-19. For example, the Chinle Service Unit deployed care coordinators with tablets to the homes of high-risk individuals in remote rural homes on the Navajo Nation to facilitate video-visits.
The Uniting Tribal Nursing Homes in Excellence collaborative of Tribal LTSS programs has been meeting regularly to share tactics and approaches to maintain the health of their residents and staff and to limit risk of exposure to COVID-19. The collaborative also presented on the CMS/ACL/IHS LTSS Webinar series hosted by the CMS Tribal LTSS Technical Assistance website. IHS and Tribal contact tracers, public health nurses, and community health representatives have worked with families to protect elders with dementia living in multi-generational homes from exposure.
(ONGOING) Action 2.H.4: Review and report on federal programs and initiatives aimed at decreasing health disparities in Alzheimer’s disease and related dementias
Lead Agency: ASPE
ASPE will convene federal partners and inventory completed and ongoing programs and initiatives to address racial and ethnic disparities in clinical care, research, and LTSS for people with dementia. ASPE will provide a report on this inventory on its website in early 2022 and will present findings at an Advisory Council meeting.
(NEW) Action 2.H.5: Improve detection, diagnosis, and care for Alzheimer’s disease and related dementia in Tribal health systems
Lead Agency: IHS
IHS will establish an Alzheimer's Grants Program with funding to from the Consolidated Appropriations Act of 2021 and based on Tribal Consultation and Urban Confer. The funds will be awarded as cooperative agreements to Tribal and UIH Programs and as program awards to IHS facilities working in close coordination with the tribes they serve. Awardees will develop comprehensive and sustainable approaches to address AD/ADRD, including detection, diagnosis, assessment, management, and support for caregivers, and will create best practice models for replication by others. The remaining funds will support these efforts with training and technical assistance in the detection, diagnosis, and management of dementia in primary care, support for caregivers, increased awareness and recognition of dementia in Tribal communities, and development of data resources using the clinical data available through the IHS.
Goal 3: Expand Supports for People with Alzheimer’s Disease and Related Dementias and Their Families
People with AD/ADRD and their families and caregivers need supports that go beyond the care provided in formal settings such as doctors’ offices, hospitals, or nursing homes. Families and other informal caregivers play a central role. Supporting people with AD/ADRD and their families and caregivers requires giving them the tools that they need, helping to plan for future needs, and ensuring that safety and dignity are maintained. Under this goal, the Federal Government and partners will undertake strategies and actions that will support people with the disease and their caregivers.
Strategy 3.A: Ensure Receipt of Culturally and Linguistically appropriate Education, Training, and Support Materials
Caregivers often report that they feel unprepared for some of the challenges of caring for a person with AD/ADRD; for example, caring for a person with sleep disturbances, behavioral changes, in need of physical assistance, or with advanced dementia can be an enormous challenge. Giving caregivers the information and training that they need in a culturally and linguistically appropriate manner helps them better prepare for these and other challenges. The actions to achieve this strategy include identifying and addressing areas of training and educational needs, creating culturally and linguistically appropriate materials, and distributing these materials widely to caregivers.
(UPDATED) Action 3.A.1: Distribute federally-developed educational materials
Lead Agencies: NIA, ACL
Partners: ADEAR, public partners
NIA’s ADEAR Center continues to update and offer free information in English and Spanish on AD/ADRD to caregivers in print and online, as well as through its information and referral helpline, a weekly email alert specifically on caregiving issues, and social media.
For more information, see:
ACL’s NADRC develops and makes available resources in support of both paid and unpaid caregivers. Examples of such resources includes, but are not limited to:
- Guide to Billing Codes for Dementia Services
- Intellectual and Developmental Disabilities and Dementia: Practical Strategies for Professionals
- Handbook for Helping People Living Alone with Dementia Who Have No Known Support
- Working Together: How Community Organizations and First Responders Can Better Serve People Living with Dementia
- Disaster Planning Toolkit for People Living with Dementia
The library of NADRC-developed and ACL grantee-developed resources for persons living with AD/ADRD and their caregivers can be found online.
For more information, see:
- https://nadrc.acl.gov/details?search1=151#result
- https://nadrc.acl.gov/details?search1=155#result
- https://nadrc.acl.gov/details?search1=157#result
- https://nadrc.acl.gov/details?search1=169#result
- https://nadrc.acl.gov/details?search1=232#result
IA2, a recipient of the HBI support, and Northwest Portland Area Indian Health Board are developing, tailoring, and disseminating AD/ADRD materials and resources to AI/AN communities.
For more information, see:
Also see Action 1.E.3 for updates on the Caregiving and SCD infographics, and Action 1.E.3 for information on CDC’s contribution to the Public Health Perspectives on the Family Care Gap textbook.
(UPDATED) Action 3.A.2: Utilize health information technology for caregivers and persons with Alzheimer’s disease and related dementias
Lead Agency: AHRQ
Partners: NIA, VA
Reports from the National Research Council have reinforced the need for HIT applications for caregivers, as well as people with AD/ADRD and providers. Many opportunities exist for using technology to support people with AD/ADRD and their caregivers. Opportunities include assistance with reminders, communications, and monitoring. AHRQ has awarded three grants for integrating information and communication technology to facilitate aging in place.
One grant (1P50HS019917) was awarded and used to create Elder Tree, a suite of electronic services to support older adults and their caregivers. The study was a randomized control trial of adults age 65 and older and their caregivers who had experienced challenges to aging in place. Control group participants were provided usual sources of information and communication, while the intervention group was given access to Elder Tree for 18 months. Findings suggested a positive effect for older adults who are heavy users of health services when they used Elder Tree. Analyses indicated that the system reduced the risk of falls and depression, and improved quality of life and social support for users. The Elder Tree tool is currently being evaluated. So far, 400 people have been recruited to use the suite, and participants were surveyed after use. An analysis is currently underway, and results will be available soon. Elder Tree has been successfully disseminated to 57 counties in Wisconsin and continues to expand.
Another grant (5R18HS022836) was awarded to evaluate use of remote sensory technology to help manage persons with AD/ADRD and to study the impact on ability of caregivers to manage a family member with AD/ADRD. So far, 60 caregivers have been recruited, out of a goal of 100, and systems are in the process of being installed and caregivers trained.
Finally, a grant (2R21HS026571) was awarded to evaluate the clinical integration of an AD/ADRD support application that provides education, supportive resources, and a platform to share real-time patient-related information with the care team from homes or community settings. The research team was modified based on feedback from stakeholders and is currently implementing the solution into the clinical environment.
For more information, see:
- https://digital.ahrq.gov/ahrq-funded-projects/bringing-communities-and-technology-together-healthy-aging
- https://digital.ahrq.gov/ahrq-funded-projects/examining-clinical-workflow-and-outcomes-integrating-health-information
NIA collaborated with ACL to issue a SBIR FOA that addresses the fundamental need for the development of technologies that enhance caregiver training and address the financial and legal aspects of caregiving. NIA and ACL published this funding opportunity to encourage and fund research and development of technology and tools for the currently under-developed market serving caregivers and their family members suffering from AD/ADRD. In their collaboration, NIA and ACL seek to stimulate research and development of technology and tools that adapt to a range of levels of expertise/experience, specific care demands, and needs of family caregivers.
For more information, see:
The VA Caregiver Center, located at the Memphis VA Medical Center and supported by the VA’s Caregiver Support Program, implemented a supportive texting intervention for caregivers of veterans with dementia. Caregivers receive information about managing dementia behaviors and their own stress and coping through Annie, the VA’s text messaging platform managed by the Office of Connected Care. National roll-out of the protocol occurred in March 2021.
Strategy 3.B: Enable Family Caregivers to Continue to Provide Care while Maintaining Their Own Health and Well-Being
Even though unpaid caregivers usually prefer to provide care in their home or other community settings, often the round-the-clock care needs of the person with AD/ADRD proves very challenging. While they are providing care, supports for families and caregivers can help lessen feelings of depression and stress and help delay or avert institutional care. The actions below will further support unpaid caregivers by identifying their support needs, developing and disseminating interventions, giving caregivers information they need, particularly in crisis situations, and assisting caregivers in maintaining their health and well-being.
(UPDATED) Action 3.B.1: Develop and disseminate evidence-based interventions for people with Alzheimer’s disease and related dementias and their caregivers
Lead Agency: NIA
Partners: AHRQ, CMS, CDC, ACL
In 2019, NIH funded a new effort called the IMPACT Collaboratory to meet the urgent public health need to deliver high-quality, evidence-based care to people living with dementias and their caregivers. Through this effort, researchers develop and test care interventions in real-world settings such as hospitals, assisted living facilities, nursing homes, and adult daycare centers. In general, a pragmatic clinical trial means that participants are enrolled as part of a real-world setting rather than selected from a broader community based on narrowly defined criteria. The IMPACT project will bolster the nation’s capacity to conduct pragmatic clinical trials of interventions, embedded within health care systems for people living with dementia and their caregivers. IMPACT supports pilot projects that have the potential to inform the design of larger scale pragmatic trials. To date, the Collaboratory has supported multiple pilot projects and career development awards for researchers from varied disciplines. The IMPACT Collaboratory continues to expand in 2021 with multiple career development and pilot and demonstration funding opportunities to support investigators interested in conducting Embedded Pragmatic Clinical Trials in health care systems, assisted living facilities, adult day programs, emergency departments, hospitals, home care, nursing homes, and other settings.
With its previously established network of partnering health care systems, the IMPACT Collaboratory proved crucial in helping researchers quickly pivot and effectively respond to the COVID-19 pandemic with new studies. Given its goal of finding novel ways to deliver high-quality, evidence-based care to people living with dementia and their caregivers, the Collaboratory’s infrastructure was primed and ready to rapidly support the development of and conduct studies to assess the pandemic’s acute impact on older adults, particularly people living with dementia. One outcome was an analysis showing that cognitive impairment is linked to elevated death rates in nursing home residents with COVID-19. In response to COVID-19 and its devastating effects on people living with dementia and their families and caregivers, NIA awarded the IMPACT Collaboratory with several supplements.
In addition, through two NIA supplemental awards, Designing a Real Time COVID-19 Vaccination Adverse Event Monitoring System for Nursing Home Residents and Monitoring Medicare Beneficiaries' Response to COVID Vaccines, researchers are supporting the development of data-sharing and reporting systems and data infrastructure to monitor the effects of the COVID-19 vaccines administered to frail older adults, including those living with dementia. These initiatives will provide near real-time insight into the use, effects, and outcomes related to use of COVID-19 vaccines among this population.
In further response to the COVID-19 pandemic, NIA supported more than 30 administrative supplements to address the challenges experienced by persons living with dementia and their care partners.
For more information, see:
- https://impactcollaboratory.org/
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-19-009.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-21-009.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-21-018.html
- https://projectreporter.nih.gov/project_info_description.cfm?aid=9774609&icde=51150896&ddparam=&ddvalue=&ddsub=&cr=1&csb=default&cs=ASC&pball=
- https://impactcollaboratory.org/grants-program/pilot-grant-awardees/
- https://www.nia.nih.gov/research/dbsr/edward-r-roybal-centers-translational-research-behavioral-and-social-sciences-aging
- https://www.roybalniaresearchcenters.org/
NIA expanded its Edward R. Roybal Centers for Translational Research to include four new AD/ADRD-specific Roybal Centers for translational intervention development research for AD/ADRD care provider support. The purpose of the Roybal Centers is to develop behavioral interventions that improve the health, well-being and/or capacity of individuals and/or systems that provide care to persons with AD/ADRD. Specifically:
- U. Penn Roybal: Supports the development of interventions to help persons with dementia receive much-needed palliative care services, as well as to help their family caregivers.
- U. Rochester Roybal: Seeks to develop behavioral interventions that promote social connectedness, particularly among family caregivers of persons with AD/ADRD.
- Emory U. Roybal: Supports the development of interventions to improve the role-mastery of informal caregivers of persons living with AD/ADRD.
- Oregon Roybal: Seeks to leverage innovations in technology to improve dementia care provider support.
For more information, see:
- https://grants.nih.gov/grants/guide/RFA-files/RFA-AG-19-007.html
- https://www.nia.nih.gov/research/dbsr/edward-r-roybal-centers-translational-research-behavioral-and-social-sciences-aging
- https://reporter.nih.gov/search/m-zoWEyxzkOMTlkCABpxEw/projects?shared=true
- https://www.roybalniaresearchcenters.org/
NIA’s ADEAR Center continues to update and offer free information in English and Spanish on AD/ADRD caregiving in print and online, as well as through its information and referral helpline, a weekly email alert specifically on caregiving issues, and social media.
Additionally, the 2020 National Research Summit on Care, Services, and Supports for Persons with Dementia and their Caregivers provided a platform to disseminate evidence-based interventions for people with AD/ADRD and their caregivers. Released in December 2020, the final report summarizes research gaps and opportunities, which address areas of scientific inquiry that hold promise for propelling advances in policy, practice, and care to improve the lives of persons who are affected by AD/ADRD and their care partners and encompass a broad swath of topics related to care and services. For example, there is strong evidence of profound disparities in dementia care among subpopulations most affected by AD/ADRD. However, this evidence base is incomplete and new research is needed to explore effects on health and receipt of care in subpopulations that are less well understood (e.g., minoritized populations and those who live alone with dementia).
For more information, see:
- https://www.nia.nih.gov/2020-dementia-care-summit
- https://aspe.hhs.gov/national-research-summit-care-services-and-supports-persons-dementia-and-their-caregivers
- https://www.nia.nih.gov/sites/default/files/2021-01/DementiaCareSummitReport.pdf
In addition to these activities, NIA released several FOAs in the past year that call for research to improve the care of persons living with AD/ADRD and their caregivers and continued to solicit research in this area under several active FOAs that were issued in previous years.
For more information, see:
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-18-056.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-18-057.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-20-026.html
- https://grants.nih.gov/grants/guide/pa-files/par-21-099.html
- https://grants.nih.gov/grants/guide/pa-files/par-21-100.html
- https://www.nia.nih.gov/health/alzheimers/caregiving
ACL’s grant programs to states and communities are designed to develop and enhance dementia-capable HCBS systems. All grantees are required to include evidence-based or evidence-informed interventions in their funded programs. In 2019, 29 programs were funded, and in 2020, 16 programs were funded bringing the program total to 108 state and community programs funded in the United States and its territories since 2014. Subject to appropriations, ACL anticipates continuing the programs to increase the availability of evidence-based interventions across the country.
ACL’s ADPI and NADRC continue to support the translation and implementation of dementia-specific evidence-based interventions in states and communities across the Nation. ACL programs support the implementation of 17 evidence-based interventions, preparing some to be taken to scale across the Nation. For example, NIA funded the research behind interventions SAVVY Caregiver, REACH-II, and Care of Persons with Dementia in their Environments, which are ACL-funded provider pilot programs that enable formal and informal caregivers and people living with dementia to benefit from the intervention.
For more information, see:
- http://nadrc.acl.gov
- https://nadrc.acl.gov/details?search1=110#result
- https://nadrc.acl.gov/details?search1=140#result
The U.S. Department of Defense (DoD) has funded REACH Hope, Supporting Caregivers of Veterans with TBI and Alzheimer’s Dementia/Mixed Dementia: The REACH Hope Behavioral Intervention, to assist caregivers of veterans who are living with TBI and dementia. The 3-year study, August 2020-August 2023, combines two behavioral interventions, Resources for Enhancing All Caregivers Health in the VA (REACH-VA) and the DoD’s Virtual Hope Box mobile App, to support caregivers one-on-one in real-time and as-needed. The study is being conducted by investigators at the VA Caregiver Center at the Memphis VA Medical Center, which is funded by the VA’s Caregiver Support program, and investigators at Virginia Commonwealth University.
CDC has also developed guidance and numerous documents for the public to keep persons with dementia and their caregivers safe from COVID-19. This guidance addresses how both formal and informal caregivers can maintain their own health and the health of the person with dementia for whom they are caring. Specific guidance was also developed to address COVID-19 circumstances in nursing homes, assisted living, and memory care units.
For more information see:
- https://www.cdc.gov/coronavirus/2019-ncov/hcp/long-term-care.html
- https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/caregivers-dementia.html
- https://www.cdc.gov/coronavirus/2019-ncov/hcp/nursing-home-long-term-care.html
- https://www.cdc.gov/coronavirus/2019-ncov/hcp/assisted-living.html
- https://www.cdc.gov/coronavirus/2019-ncov/hcp/memory-care.html
(UPDATED) Action 3.B.2: Provide effective caregiver interventions through Alzheimer’s disease and related dementias-capable systems
Lead Agency: ACL
ACL’s grant programs to states and communities are designed to develop and enhance dementia-capable HCBS systems. ACL programs have supported the implementation of 15 evidence-based interventions, preparing some to be taken to scale across the Nation. In 2020, 16 programs, including four Tribal communities, and, as of August 2021 an additional 11 state and community programs and one Tribal program were funded bringing the program total to 117 state, community and Tribal programs funded in the United States and its territories since 2014. Subject to appropriations, ACL anticipates continuing the program to increase the availability of evidence-based interventions across the country.
For more information, see:
(ONGOING) Action 3.B.3: Collaborate to share information on long-term services and supports with Tribal providers
Lead Agency: ACL
Partners: IHS, CMS
HHS uses multiple mechanisms to share information on LTSS and care of the person and family with AD/ADRD with Tribal providers. IHS, ACL, and CMS will develop a joint website on LTSS for Tribal providers. IHS conducts presentations on LTSS for people with AD/ADRD at Indian Country conferences, including the Older Americans Act (OAA) Title VI annual conference, and conferences for Tribal Health Directors and Planners (NIHB), Tribal Leaders (National Council on American Indians), and Tribal Elders (National Indian Council on Aging [NICOA]). IHS and ACL host joint webinars on addressing the service and supports needs of persons with AD/ADRD. Dissemination of dementia-specific information through presentations occurs at Indian Country meetings and webinars.
In 2020, ACL introduced a new grant program, “ADPI: Dementia Capability in Indian Country”. The program is designed to bring culturally-appropriate AD/ADRD training and education to Indian Country. Four tribes received 3-year awards in August 2020.
(ONGOING) Action 3.B.4: Continue to promote use of the National Alzheimer’s Call Center to provide information, advice, and support to people with dementia or their caregivers
Lead Agency: ACL
Partners: private partners
ACL continues to provide funding toward and promote use of the National Alzheimer’s Call Center to provide information, advice, and support about AD/ADRD. The Call Center provides 24-hour access, 7 days a week via a toll-free number (1-800-272-3900). Support varies from simple referrals to crisis intervention. Complex and crisis calls are handled by master's level social workers and counselors who provide reflective listening, problem solving, education, action planning, and crisis intervention. The Call Center provides assistance in over 170 languages.
(ONGOING) Action 3.B.5: Make behavioral symptom management education and training available to caregivers
Lead Agency: ACL
Partner: CMS
ACL continues to expand efforts to develop more dementia-capable LTSS systems designed to meet the needs of AD/ADRD caregivers. ACL requires that all ADPI community grants include behavioral symptom management and expert consultations to support caregivers in their programs.
(UPDATED) Action 3.B.6: Adapt and implement Resources for Enhancing Alzheimer’s Caregivers’ health in Tribal communities
Lead Agency: IHS
Partners: ACL, VA, University of Tennessee Health Sciences Center
The initial phase was completed with 80 REACH certified caregiver support coaches in 56 Tribal communities, serving at least 55 caregivers as of February 2018. The second phase of the effort is focused on implementing strategies to increase penetration of REACH in the communities where there are certified coaches and building additional, sustainable options for evidence-based caregiver support services. IHS continues work developing the Extension for Community Healthcare Outcomes project model to support Caregiver Coaches in Tribal Communities and to identify additional training for caregiver support through public health nursing. IHS collaborated with the VA Greater Los Angeles GRECC in the development of the IHS ABCD (Addressing Behavioral Challenges in Dementia) training targeted at Public Health Nurses who provide support for caregivers of persons living with dementia.
In 2020, ACL-funded grantees to train REACH certified caregiver support coaches to deliver the intervention to Aleutian Pribilof Islands Association members. The grantee provides Health Services (primary care, behavioral health, community wellness, and prevention) in four communities (Atka, Nikolski, St. George, and Unalaska) across the Aleutian and Pribilof Islands.
For more information, see:
(ONGOING) Action 3.B.7: Develop and disseminate information to caregivers on Alzheimer’s disease and related dementias and caregiving
Lead Agency: CDC
Partner: ACL
CDC developed a downloadable care planning tool to assist people with AD/ADRD and their caregivers. Care plans can reduce emergency department visits, hospitalizations, and improve overall medical management for people with a chronic health condition, like AD/ADRD, resulting in better quality of life for all care recipients.
CDC has developed a series of web features and podcasts on topics including helping people with AD/ADRD and their caregivers stay physically active, developing care plans for older adults and their caregivers, and the truth about aging and dementia.
For more information, see:
- https://www.cdc.gov/aging/caregiving/pdf/Complete-Care-Plan-Form-508.pdf
- https://www.cdc.gov/features/caregivers-month/index.html
- https://www.cdc.gov/aging/publications/podcasts.htm
Annually ACL, through the NADRC and its grant programs, continues to develop and make available web content on issues relevant to paid and unpaid caregivers. In addition to hosting ten webinars on a broad range of AD/ADRD topics, the NADRC has developed several resources:
- Handbook for Helping People Living Alone with Dementia Who Have No Known Support
- Disaster Planning Toolkit for People Living with Dementia
- Working Together: How Community Organizations and First Responders Can Better Serve People Living with Dementia
- Guide to Billing Codes for Dementia Services
For more information, see:
In Fall 2020, ACL and the NADRC hosted a three-part COVID-19 webinar series to highlight ways in which organizations pivoted to support people living with dementia and their caregivers. The series included presentations on innovative approaches to ensure people living with dementia continued to receive needed services through traditional means of in-person delivery, as well as using technology to deliver services. The expectation is that when all in-person programs are able to resume, the new normal is likely to include hybrid programs that include both in-person and virtual opportunities.
For more information, see:
- https://nadrc.acl.gov/details?search1=20210224014846#result
- https://nadrc.acl.gov/details?search1=20210225020522#result
- https://nadrc.acl.gov/details?search1=20210225025052#result
- https://acl.gov/COVID-19
(ONGOING) Action 3.B.8: Develop a program to support enhanced financial literacy and preparedness of family caregivers
Lead Agency: ACL
Partners: private partners, NIA
Family caregivers often lack adequate information and resources to properly manage the financial concerns of their loved ones. In 2018, ACL introduced a new program to address the need to advance understanding financial literacy of family caregivers. The program addresses that need through the development and testing of new interventions, as well as identification and dissemination of best practices.
See Action 3.A.2 for information on an NIA/ACL SBIR FOA that addresses the fundamental need for the development of technologies that enhance caregiver training and address the financial and legal aspects of caregiving.
(NEW, COMPLETED) Action 3.B.9: Provide caregivers of veterans living with dementia with information about VA Caregiver Support Program resources available to them
Lead Agency: VA
To provide information to caregivers of veterans living with dementia about VA Caregiver Support Program resources available to them, the VA Caregiver Support Leadership Council Education Committee sponsored three monthly education sessions in FY 2021. Sessions were scheduled for June 30, August 4, and September 8. Caregivers could attend by videoconference or telephone. Caregivers of veterans living with dementia may contact their local VA medical facility’s Caregiver Support staff for more information.
For more information, see:
Strategy 3.C: Assist Families in Planning for Future Care Needs
The vast majority of people do not think about or plan for the LTSS they will need until they experience a disability or AD/ADRD. Many Americans incorrectly believe that Medicare will cover most of the costs of these supportive long-term care services like nursing home care and HCBS. Unfortunately, by the time care is needed, it is difficult to get coverage in the private long-term care insurance market and financing options are limited. Educating people about their potential need for LTSS and the significant advantages of planning ahead for these services encourages timely preparation. Planning ahead can help ensure that individuals with AD/ADRD receive care in the setting they prefer, preserve individual and family assets, and maintain dignity.
(COMPLETED) Action 3.C.1: Understand the risks and costs of cognitive impairments
Lead Agency: ASPE
ASPE completed a project to understand the expected lifetime risks and costs of cognitive impairment, including estimates of the value of informal care. The possibility of becoming severely cognitively impaired is among the most consequential risks facing older adults and their families. In addition to the emotional and physical toll associated with dementia, the financial consequences can be overwhelming, as many patients require expensive paid care. Projections of future care needs and costs are difficult because the older population is changing in ways that will likely shape the course of cognitive impairment. This study uses the Dynamic Simulation of Income Model (DYNASIM) to project the risk and costs of severe cognitive impairment at older ages over the coming decades. Using multiple data sources and sophisticated econometric techniques, DYNASIM simulates the future population and its characteristics, projecting financial resources, disability status, medical conditions, cognitive status, and use of LTSS. Unlike a lot of past research, this study will show how severe cognitive impairment and associated costs vary across the population. The final report was posted on the ASPE website in January 2021.
For more information see:
- https://aspe.hhs.gov/reports/risk-costs-severe-cognitive-impairment-older-ages-literature-review-projection-analyses
- https://aspe.hhs.gov/reports/risk-costs-severe-cognitive-impairment-older-ages-key-findings-our-literature-review-projection
(COMPLETED) Action 3.C.2: Understanding the availability of caregivers for individuals with long-term services and supports needs
Lead Agency: ASPE
ASPE completed a project to understand widely stated concerns about an impending shortage of informal caregivers and a resulting increase in unmet needs for care as the large Baby Boomer cohort enters retirement ages. The project compiled existing evidence in the literature and explored the implications of population aging and other changing demographic characteristics for future met and unmet care needs and reliance on Medicaid LTSS. The final report was posted on the ASPE website in December 2020.
For more information see:
(ONGOING) Action 3.C.3: Empowering people to make better informed health care decisions
Lead Agency: CMS
CMS’s Care Compare website, a streamlined redesign of eight existing CMS health care compare tools, provides a single user-friendly interface that patients, caregivers, and consumers can use to make informed decisions about health care based on cost, quality of care, volume of services, and other data.
For more information, see:
(ONGOING) Action 3.C.4: Expand availability of care planning tools for people with dementia
Lead Agency: CDC
CDC developed a downloadable care planning tool to assist people with AD/ADRD and their caregivers. Care plans can reduce emergency department visits, hospitalizations, and improve overall medical management for people with a chronic health condition, like AD/ADRD resulting in better quality of life for all care recipients.
For more information, see:
(NEW) Action 3.C.5: Model future expenditures on long-term services and supports and use of informal caregivers
Lead Agency: ASPE
As the United States population ages, a larger proportion of individuals will likely need and use LTSS. Much of this support is provided by informal (i.e., unpaid) caregivers. For those that need paid LTSS, most Americans pay out-of-pocket. Some may do so until their personal resources are exhausted, and then rely on the Medicaid safety net. Reliance on Medicaid for those with little income or limited assets may result in increased federal and state spending for LTSS. As such, there is a pressing need to understand the current cost of long-term care, national expenditures on LTSS, and future projections of the availability of informal caregivers. This project will build off previous ASPE work modeling LTSS needs and expenditures, as well as work that explores how key demographic changes will affect the supply of informal caregivers for older Americans. One of the primary goals of the project is to provide current and improved estimates of the value of informal caregiving and diversity in caregiving provision, as well as projections that show how changing demographics could affect older Americans need for LTSS, the supply of future caregivers and Medicaid spending.
Strategy 3.D: Maintain the Dignity, Safety and Rights of People with Alzheimer’s Disease and Related Dementias
People with AD/ADRD are particularly vulnerable to financial exploitation, physical or emotional abuse, and neglect both at home and in care facilities. Reports of elder abuse are handled by state Adult Protective Services (APS), which investigate allegations, provide protective services, and refer cases to law enforcement when appropriate. Not all APS programs cover residents of long-term care facilities. State survey and certification agencies receive funding from CMS to survey Medicare or Medicaid-certified nursing facilities and to investigate abuse complaints, among others, in these facilities. State licensing agencies may investigate complaints of abuse in other types of facilities, such as assisted living. State long-term care ombudsmen programs advocate for residents of nursing homes and other adult care facilities, and work to resolve complaints on behalf of residents, including those related to abuse, neglect, and exploitation. The Actions below will help ensure that people with AD/ADRD have their dignity, safety, and rights maintained.
(ONGOING) Action 3.D.1: Monitor, report and reduce inappropriate use of antipsychotics in nursing homes
Lead Agency: CMS
Partners: ACL, NORC
The National Partnership to Improve Dementia Care in Nursing Homes is committed to improving the quality of care for individuals with dementia living in nursing homes. The National Partnership has a mission to deliver health care that is person-centered, comprehensive and interdisciplinary with a specific focus on protecting residents from being prescribed antipsychotic medications unless there is a valid, clinical indication and a systematic process to evaluate each individual’s need. While there are circumstances where clinical indications for the use of antipsychotic medications are present, more work needs to be done to ensure these indications are accurate.
For more information, see:
- https://store.samhsa.gov/product/Guidance-on-Inappropriate-Use-of-Antipsychotics-Older-Adults-and-People-with-Intellectual-and-Developmental-Disabilities-in-Community-Settings/PEP19-INAPPUSE-BR
- https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/National-Partnership-to-Improve-Dementia-Care-in-Nursing-Homes
CMS continues the Civil Money Penalty Reinvestment Program (CMPRP), an effort to drive improvements in quality of life and quality of care for nursing home residents. CMPRP is funded by the federal portion of civil monetary penalty funds to conduct activities that support and protect nursing home residents. This program builds on other CMS initiatives such as the National Partnership.
For more information, see:
- https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/LTC-CMP-Reinvestment.html
- https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/National-Partnership-to-Improve-Dementia-Care-in-Nursing-Homes.html
- https://www.cms.gov/Outreach-and-Education/Outreach/NPC/National-Provider-Calls-and-Events.html
(UPDATED) Action 3.D.2: Incorporate elder abuse awareness into Aging Network activities
Lead Agency: ACL
Partners: NIA, private partners
ACL continues to expand awareness and detection of elder abuse and neglect among the population of people with dementia. ACL encourages the Eldercare Locator and other Aging Network and prevention program providers to become knowledgeable about warning signs of abuse. These providers will also disseminate information on elder abuse, with a particular focus on the vulnerable population of people with AD/ADRD.
In 2021 the NADRC hosted a webinar, entitled Elder Abuse in People Living with Dementia: Prevention, Detection, and Intervention, in which a physician and an attorney discussed indicators that should raise concern, provided practical tips on when and how to intervene, and pay particular attention to the complicated issue of capacity.
For more information, see:
(UPDATED) Action 3.D.3: Translate and disseminate information on abuse of people with dementia
Lead Agency: ACL
Partners: NIA, DoJ, private partners
ACL, NIH, and the U.S. Department of Justice (DoJ) have funded research focused on the abuse, neglect, and exploitation of older adults. HHS will work with the private sector to translate these findings into educational materials and resources as well as other intervention programs related to the abuse of people with AD/ADRD.
ACL continues to fund programs designed to address elder abuse. National Center on Elder Abuse is funded by ACL and, through their programs, addresses all facets of elder abuse, including the abuse of individuals living with dementia.
For more information, see:
NIA produces online and print content on Elder Abuse, including a 2019 infographic Spotting the Signs of Elder Abuse, to educate the public and disseminate information about identifying and addressing types of elder abuse and dealing with caregiver stress.
NIA is also currently supporting and actively soliciting new research in this area via current FOAs. For example, NIA recently published RFA-AG-22-024: Primary Care-Based Screening and Intervention Development for Prevention of Abuse in Older and Vulnerable Adults in the Context of AD/ADRD, which is soliciting research that can lead to the development of evidence-based primary care screening tools and behavioral interventions to prevent abuse in at-risk older and vulnerable adults with MCI and AD/ADRD and their families. With RFA-AG-22-020: Triadic Interactions in Clinical Encounters Involving People with AD/ADRD, Clinicians, and Care Partners, NIA invited research on clinician screening tools for abuse and behavioral interventions for unhealthy caregiving relationships. NOT-AG-20-039: Notice of Special Interest: Fundamental and Translational Research on Decision Making in Aging and/or AD/ADRD invited research focused on social and other factors that render older adults vulnerable to financial exploitation and other forms of mistreatment and abuse. Per NOT-AG-18-057: Notice to Specify High-Priority Research Topic for PAR-19-070 and PAR-19-071, NIA is soliciting research on risk, protective, and resilience factors related to elder mistreatment and interventions to prevent or detect elder mistreatment in informal care settings for individuals with AD/ADRD.
For more information, see:
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-22-020.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-22-024.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-18-057.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-20-039.html
- https://www.nia.nih.gov/health/elder-abuse
DoJ launched a guardianship page on the Elder Justice Initiative (EJI) website in July 2021 for the general public, providing information and resources related to guardianship itself and to abuse perpetrated by guardians. The web page features four sections: an overview, least restrictive options, key concepts and resources, and mistreatment and abuse by guardians and other fiduciaries.
For more information, see:
DoJ’s National Institute of Justice (NIJ) maintains a web page featuring NIJ-funded elder abuse research entitled Overview of Elder Abuse, and another page specifically on financial exploitation entitled Financial Exploitation of the Elderly.
For more information, see:
- https://nij.ojp.gov/topics/articles/overview-elder-abuse
- https://nij.ojp.gov/topics/articles/financial-exploitation-elderly
DoJ’s EJI website hosts the Elder Abuse Resource Roadmap dedicated to identifying where to report financial exploitation in addition to information resources on a variety of financial exploitation topics. DoJ’s EJI website also hosts an elder justice research web page featuring foundational articles, some of which address elder abuse and dementia.
For more information, see:
- https://www.justice.gov/elderjustice/roadmap
- https://www.justice.gov/elderjustice
- https://www.justice.gov/elderjustice/research-related-literature
- https://www.justice.gov/elderjustice/foundational-articles
In addition, EJI’s renowned webinar series features several webinars on this topic presented by elder justice experts:
- The Neuroscience Behind Financial Scams
- Responding to Elder Abuse Victims with Alzheimer’s Disease or Other Dementias
- Increasing Access to Capacity Assessments via New Technologies
- Digging Deeper: When Consent is Not Consent
- Assessing Cognitive Capacity in Elder Abuse Cases
Webinars scheduled for the upcoming year include:
- Innovations in Guardianship: Maximizing Autonomy and Ensuring Accountability
- Identifying and Prosecuting Power of Attorney Abuse
- Culturally Sensitive Counseling for Older Adult Survivors
For more information, see:
- https://www.justice.gov/elderjustice/video/neuroscience-behind-financial-scams
- https://www.justice.gov/elderjustice/video/responding-elder-abuse-victims-alzheimer-s-disease-or-other-dementias
- https://ovcttac.adobeconnect.com/px6tzz3q5y94/
- https://www.justice.gov/elderjustice/video/digging-deeper-when-consent-not-consent
- https://www.justice.gov/elderjustice/video/assessing-cognitive-capacity-elder-abuse-cases
The EJI supported the development of Finding the Right Fit: Decision-Making Supports and Guardianship in collaboration with the National Center for State Courts. This online training is designed to assist individuals in exploring ways to help someone who may need assistance in making decisions with informal supports, legal options, and/or adult guardianship. Finding the Right Fit provides a broad overview of decision making supports and guardianship that is not specific to state laws or rules.
For more information, see:
(ONGOING) Action 3.D.4: Improve the ability of legal services to address the needs of people with Alzheimer’s disease and related dementias
Lead Agency: ACL
Partners: NLRC, legal assistance developers
ACL has a number of related activities underway to improve legal services for people with AD/ADRD. The ACL National Legal Resource Center (NLRC) website includes a special section addressing advance planning and end-of-life issues, a resource for legal and aging/disability service professionals and family caregivers assisting people with AD/ADRD or other causes of diminished capacity.
ACL-funded state and community grants programs include pilot programs designed to make dementia-capable legal services available to persons with dementia and their caregivers. Program participants are providing dementia training to legal services providers, as well as implementing voucher programs to aide in advance planning.
ACL grants to states and communities include pilot programs designed to make dementia-capable legal services available to persons with dementia and their caregivers. Program participants are providing dementia training to legal services providers, as well as implementing voucher programs to aide in advance planning.
In 2018, the NADRC partnered with the American Bar Association Commission on Law and Aging to develop The Handbook for Helping People Living Alone with Dementia Who Have No Known Support. Among other things it provides practical guidance as well as tools for helping a person living alone who does not have informal supports. The Handbook includes practical strategies for identifying people who are living alone without support, assessing risk, building trust, identifying family and friends willing to help, determining decision making capacity, options for helping the person maintain their independence, and the basics of guardianship or conservatorship.
For more information, see:
(ONGOING) Action 3.D.5: Educate law enforcement and other first responders about interacting with individuals with Alzheimer’s disease and related dementias
Lead Agency: DoJ
Partner: ACL
DoJ continues to educate law enforcement and public safety professionals about how to interact appropriately with missing persons with AD/ADRD and to provide current information and resources to help law enforcement agencies and the communities they serve. This education will include how to prevent persons with AD/ADRD from wandering and becoming lost, as well as information on locating those who do wander and become lost. The training and resources are provided through projects funded by the Office of Justice Programs’ Bureau of Justice Assistance.
ACL grantees are using grant funds to engage with and train law enforcement and other first responders. One grantee created a series of well-received law enforcement training videos to address wandering, driving, and encountering disoriented individuals on “house calls”. Another grantee has developed Gun Violence Restraining Order training and partnered with the Deputy City Attorney for its delivery.
For more information, see:
The EJI continues its commitment to ensuring law enforcement has the training and tools to robustly and appropriately respond to victims of elder abuse, including persons with AD/ADRD. For example, EJI currently hosts relevant resources on the law enforcement web page, including:
- Safe Return: Alzheimer’s Disease Guide for Law Enforcement (Alzheimer’s Association)
- A Booming Problem: Alzheimer’s, Dementia, and Elder Abuse (DoJ Office of Community Oriented Policing Services)
- Approaching Alzheimer's: First Responder Training Program (Alzheimer’s Association)
- Communicating with Someone with Dementia (Alzheimer’s Association)
For more information, see:
- https://www.justice.gov/elderjustice/law-enforcement-1
- http://www.alz.org/national/documents/SafeReturn_lawenforcement.pdf
- http://cops.usdoj.gov/html/dispatch/05-2015/alzheimers_dementia_elder_abuse.asp
- http://www.alz.org/care/alzheimers-first-responder.asp
- https://www.alz.org/help-support/caregiving/daily-care/communications
The EJI is collaborating with the Federal Bureau of Investigation to support the development of curriculum for forensic interviewing with older adults. This is an advanced forensic interviewing certificate curriculum for established forensic interviewers with previous basic certification and extensive experience in forensic interviewing and/or working with older adults and adults with AD/ADRD. The advanced forensic interview certificate curriculum is designed to support forensic interviewers to gather information from alleged victims in the most reliable and legally defensible manner. From this in-depth curriculum, supplemental curricula for front line responders and for elder justice professionals will also be developed. The curricula are scheduled for completion in October 2021. This project builds upon a previous (December 2017) EJI-hosted webinar on Forensic Interviewing with Older Adults, featuring aspects of dementia as part of this training that remains available for professionals to download.
For more information, see:
The EJI is collaborating with the University of Southern California to develop The Enhanced AIM Judicial Review Tool. This is a standardized framework and pragmatic tool based upon the Abuse Intervention Model (AIM) for judges, court officers, and investigators to better evaluate and assimilate evidence related to capacity and the risk for elder mistreatment, including aspects of AD/ADRD. The tool is scheduled for completion in December 2021.
(ONGOING) Action 3.D.6: Work with communities to develop best practices for protecting people with Alzheimer’s disease and related dementias
Lead Agency: ACL
ACL, NADRC and dementia grantees continue to make the provision of dementia-specific education of first responders a priority in their funded programs. NADRC developed a guide for first responders entitled Working Together: How Community Organizations and First Responders Can Better Serve People Living with Dementia. The guide helps community organizations collaborate with first responders to better serve people living with dementia, a need increasingly recognized by first responder agencies. This guide explains why this issue is gaining attention, provides strategies for building successful partnerships, and describes the types of programs that can benefit people living with dementia. Also included are resources such as training materials, sample policies, tip sheets and more.
In addition to the guide, ACL grantees developed a training session on the basics of dementia for first responders which includes descriptions of dementia, the changes that accompany dementia (e.g., communication, behavior), and important safety and wandering issues related to dementia. Alzheimer’s San Diego created a referral form that law enforcement can use. To refer an individual or family member to Alzheimer’s San Diego for support or education. A complement to these resources is the four-part training series of videos developed by Alzheimer’s Orange County which present educational vignettes to address wandering, driving, and encountering disoriented individuals on “house calls” involving actual first responders and actors portraying people living with dementia and reminders about how to handle the interactions and any follow-up.
For more information, see:
- https://nadrc.acl.gov/details?search1=155#result
- https://nadrc.acl.gov/details?search1=196#result
- https://www.youtube.com/playlist?list=PLyUU5QfNKxwhm7NTgcmUGUsfKxs_qCb20
The EJI’s Multidisciplinary Team (MDT) Technical Assistance Center provides educational offerings and technical assistance to elder abuse MDTs on the topic of detecting and providing appropriately tailored elder abuse interventions for older adults with AD/ADRD.
For more information, see:
(NEW) Action 3.D.7: Understand the predictors and outcomes of inpatient psychiatric facility placement among people living with dementia
Lead Agency: ASPE
Some people with dementia may have severe behavioral and psychological symptoms of dementia (BPSD) such as aggression, agitation, depression, or psychosis. These symptoms are associated with hospital admission and nursing home placement in addition to caregiver distress and poor health. In some cases, individuals with BPSD require intensive care and are admitted to an inpatient psychiatric facility (IPF). Previous ASPE analyses found that dementia is a common diagnosis in IPFs. In 2008, 15% of Medicare beneficiaries receiving care from an IPF had a primary diagnosis of AD/ADRD and 25% had any AD/ADRD diagnosis. However, little is known about the care settings and events that proceed an individual’s transition to, and from, an IPF. Most Medicare beneficiaries receiving care from an IPF also received care from an inpatient setting or emergency department in the 30 days preceding their IPF admission. Readmissions after an IPF stay are common.
There are anecdotal reports about poor care experiences prior to being admitted to an IPF, or during a stay. While CMS publicly displays information on certain quality measures under the Inpatient Psychiatric Facility Quality Reporting Program on its Care Compare website, more analysis is needed on how often, and under what conditions, people with dementia are going to IPFs, including how often they are admitted to IPFs from nursing homes. Similarly, little is known about the long-term care settings where people with dementia go following an IPF stay. The purpose of this ASPE study is to analyze CMS Medicare fee-for-service (FFS) data to understand the characteristics of Medicare beneficiaries with and without dementia who use IPFs, the diagnoses and service utilization that precede psychiatric inpatient stays, and the outcomes, including health care utilization and mortality, following a stay.
(ONGOING) Action 3.D.8: Develop a supported decision making model as an alternative to guardianship
Lead Agency: ACL
ACL continues to support the National Resource Center for Supported Decision-Making (NRC-SDM) which builds on and extends the work of Quality Trust's Jenny Hatch Justice Project by bringing together vast and varied partners to ensure that input is obtained from all relevant stakeholder groups including older adults, people with IDD, family members, advocates, professionals and providers. The NRC-SDM partners bring nationally recognized expertise and leadership on SDM, representing the interests of and receiving input from thousands of older adults and people with IDD. They have applied SDM in groundbreaking legal cases, developed evidence-based outcome measures, successfully advocated for changes in law, policy and practice to increase self-determination and demonstrated SDM to be a valid, less-restrictive alternative to guardianship.
In September 2020, ACL extended its commitment to keeping supported decision making as a priority with the award of a cooperative agreement to the University of Massachusetts at Boston to implement a national Alternatives to Guardianship (AtG) Youth Resource Center. The AtG is a new initiative focused on diverting high school students with IDD away from guardianship to SDM, which allows individuals with disabilities to make decisions for themselves and choose the level of support they need from people and organizations they trust.
For more information, see:
Strategy 3.E: Assess and Address the Long-Term Services and Supports Needs of People with Alzheimer’s Disease and Related Dementias
LTSS are essential to helping people with AD/ADRD receive the assistance that they need. HCBS help people with AD/ADRD remain in their homes in the community, where many prefer to be. For those who need additional support, a residential care or nursing facility may be a better fit. Through the actions below, HHS will assess the availability and quality of services across residential settings to ensure all people with AD/ADRD receive the care they need in the setting they prefer.
(COMPLETED) Action 3.E.1: Assess utilization of home health benefits
Lead Agency: ASPE
ASPE continues to study the growth in use of the Medicare home health benefit by community-admitted users (those individuals for whom home health episodes are not preceded by a hospitalization or PAC stay). The Medicare home health payment policy for FFS has undergone several changes in the past decades. There have also been overall increases in Medicare home health utilization. Growth in utilization has been particularly strong for community-admitted users. The Medicare Payment Advisory Commission has suggested that this might be an indication that some beneficiaries are using the home health benefit as a long-term care benefit. Several alternative explanations for the growth of the community-admitted users are plausible. Indeed, a recent Academy Health meeting on PAC noted that as the American population ages, those with chronic conditions will likely cycle between PAC and intermittent need for home health benefits due to their condition. The research indicates that there are many important differences between patients based on the length of their home health care use, not just based on their source of admission. The study also found that use of the home health care benefit is changing -- use of home health aide care has declined and use of physical therapy services has increased, even for longer periods of care. CMS recently implemented a new case-mix adjustment methodology and a 30-day unit of payment for Medicare home health services on January 1, 2020. The new case-mix adjustment methodology focuses more on patients’ clinical characteristics, including taking into account patients’ admission source, and eliminates the use of therapy thresholds in determining payment. ASPE is now working on research to better understand trends in home health care use in Medicare Advantage compared to traditional Medicare.
For more information, see:
- https://aspe.hhs.gov/pdf-report/patterns-care-and-home-health-utilization-community-admitted-medicare-patients
- https://aspe.hhs.gov/reports/changes-home-health-care-use-medicare-advantage-compared-traditional-medicare-2011-2016-0
(ONGOING) Action 3.E.2: Understand contributing factors to and policy implications of nursing facility closures
Lead Agency: ASPE
In Fall 2020, ASPE began an evaluation of nursing facility closures over the last decade. Nursing facility closures can have negative effects on residents and affect access to care in this setting. Although a certain proportion of nursing facility closures is expected and may be considered an appropriate market response to poor performance or oversupply, stakeholders are concerned with recent news of increases in the number of closures and how that may limit access to necessary long-term care services in some circumstances. This study will explore the incidence rate of nursing facility closures per year over the last decade and describe factors that may be contributing to those closures. The study will contribute to HHS’s general understanding of changes in the nursing facility industry and how recent closures may impact access for the aging population.
(NEW) Action 3.E.3: Determine progress made in rebalancing Medicaid long-term care toward home and community-based services among older adults
Lead Agency: ASPE
Through this project, ASPE will assess the extent states have “rebalanced” Medicaid-funded LTSS from institutional LTSS to HCBS between 2015 and 2019. Analyses will use Transformed Medicaid Statistical Information System (T-MSIS) data to examine Medicaid LTSS expenditures that went toward HCBS and nursing facility care, as well as the use of both settings among all Medicaid LTSS users and various subgroups (e.g., older adults, younger adults with adult-onset disabilities, and individuals with IDD). Analyses will involve identifying characteristics of state LTSS programs that correlate with greater rebalancing toward HCBS and calculating transition rates from the community to nursing facilities among older adults. This project will also examine patterns of transition from the community to long-stay nursing home care over a 3 year period, controlling for use of HCBS to gauge whether some states’ use of HCBS appears to be more effective than others in preventing or postponing long-stay nursing home admissions.
(NEW) Action 3.E.4: Measure differences in medical and long-term care use and expenditures of older adults over time
Lead Agency: ASPE
Newly available data linkages between Medicaid T-MSIS (which includes both FFS claims and managed care encounter data), Medicare Advantage encounter data, and the National Health and Aging Trends Study (NHATS) longitudinal data present an opportunity for researchers to learn more about the medical and long-term care service use patterns and patient outcomes of older Americans with complex care needs, and to evaluate the effectiveness of interventions and services. This new ASPE project will follow Medicare-Medicaid dual eligible and Medicare-only respondents of the NHATS over the period of 2015-2019, to measure differences in medical and long-term care use and expenditure patterns over time. The analyses will have two separate but related focal points: (1) the impact of growth in enrollment in Medicare and Medicaid managed care plans on acute and long-term care service use patterns for both Medicare-only and Medicare-Medicaid dual eligible older adults (aged 65+); and (2) factors associated with older adults, both Medicare-only and dual eligible, transitioning from the community to long-stay nursing home care that may potentially be subject to policy interventions (e.g., supports for family caregivers).
(ONGOING) Action 3.E.5: Strengthen states’ ability to provide and sustain dementia-capable home and community-based services
Lead Agency: ACL
ACL’s ADPI program continues to make funds available to states to develop and implement dementia-capable HCBS. Through the ADPI program, states are able to pilot programs in support of persons living with AD/ADRD and their caregivers in an effort to develop evidence for sustainability post-grant funding.
In 2017, ACL rolled out its dementia-capability assessment tool for implementation through the ACL state and community grant program. The tool assesses program partners over the course of a grant to measure the improvement in dementia-capability over time. The tool is available for non-grantees on the NADRC website.
For more information, see:
- https://nadrc.acl.gov/
- https://acl.gov/news-and-events/announcements/new-funding-opportunity-alzheimers-disease-programs-initiative-adpi
- https://nadrc.acl.gov/details?search1=117#result
(UPDATED) Action 3.E.6: Fill service gaps in dementia-capable systems by expanding the availability of specialized services and supports to target previously under-served populations
Lead Agency: ACL
Partner: CMS
In 2014, ACL began funding community programs designed to fill service gaps in existing dementia-capable systems. Funded programs are required to target program activities providing effective supportive services to persons living alone with AD/ADRD, improving quality and effectiveness of services for individuals aging with IDD and AD/ADRD or those at high-risk, and delivery of behavioral symptom management training and expert consultations for family caregivers. In 2020, 16 programs, including four Tribal communities, were funded. As of August 2021, an additional 11 state and community programs and one Tribal program were funded, bringing the program total to 117 state, community, and Tribal programs funded in the United States and its territories since 2014.
For more information see:
- https://acl.gov/news-and-events/announcements/alzheimers-disease-programs-initiative-adpi-2020-awards-announced
- https://acl.gov/news-and-events/announcements/alzheimers-disease-programs-initiative-adpi-dementia-capability-0
(ONGOING) Action 3.E.7: Improve home and community-based services provided through state Medicaid waivers
Lead Agency: CMS
CMS is working with its state partners to implement a new federal investment to enhance, expand, and strengthen Medicaid HCBS. Authorized by Section 9817 of the American Rescue Plan Act of 2021, over $12 billion in increased funding is being dispersed to state for their HCBS programs between April 2021 and March 2022. These funds are being used to address existing HCBS workforce and structural issues, expand the capacity of critical services, and meet the needs of people on HCBS wait lists, and unpaid caregivers. This funding also provides states an important opportunity to enhance individual autonomy and community integration in accordance with CMS’s home and community-based settings regulation, the Supreme Court’s Olmstead decision implementation, and other state “rebalancing” efforts. CMS continues to hold webinars, national calls, and provide information to key stakeholders on a wide range of HCBS topics.
For more information, see:
- https://www.medicaid.gov/medicaid/home-community-based-services/guidance/strengthening-and-investing-home-and-community-based-services-for-medicaid-beneficiaries-american-rescue-plan-act-of-2021-section-9817-spending-plans-and-narratives/index.html
- https://www.medicaid.gov/medicaid/hcbs/training/index.html
(COMPLETED) Action 3.E.8: Understand current home care agency challenges, including the impact of COVID-19, and best practices to address them
Lead Agency: ASPE
The COVID-19 pandemic has affected home care agencies -- including home health agencies -- and their staff in several important ways. Some of these effects were entirely new and resulted directly from the pandemic. In other cases, the pandemic worsened long-standing challenges in the industry. States and the Federal Government addressed some of these issues through changes to policies, regulations, and guidance. Home care agencies also responded with changes to their own policies and practices. The purpose of this study was to understand the challenges faced by home care (including home health) agencies due to the COVID-19 pandemic and the policies and practices put into place by the Federal Government, state governments, and home care agencies themselves to mitigate these challenges through a 50-state scan and interviews with stakeholders.
Agencies identified the following challenges: lack of designation as essential workers; difficulty accessing PPE, testing and vaccines; lack of guidance; staffing shortages worsened; inconsistent training requirements; and, transportation issues and client loads decreased. Agencies used the following mitigation strategies: creative solutions to obtain PPE; implementing hazard and bonus pay; shifting to virtual hiring and caregiving; providing job and hiring flexibilities; enhancing infection control; increasing communication; and, supporting employee well-being. The most common changes implemented by home care agencies and states included: wage increases, changes to staff training, qualifications or duties, electronic communications, and paying family caregivers under the Medicaid program. The Federal Government also allowed home health services under both the Medicare and Medicaid programs to be ordered by non-physician practitioners.
For more information, see:
(UPDATED) Action 3.E.9: Expand resources to support person-centered care
Lead Agency: ACL
NCAPPS is an initiative from ACL and CMS that helps states, tribes, and territories implement person-centered thinking, planning, and practice. The NCAPPS supports the provision of technical assistance in the delivery of person-centered care, including dementia care. In May 2021, the NADRC and the NCAPPS partnered on a webinar entitled Person-Centered Goal Discovery for People Living with Dementia. The webinar presented foundational person-centered principles and provided examples of how to plan for people who are living with dementia in the community and other settings.
For more information, see:
Goal 4: Enhance Public Awareness and Engagement
Most of the public is aware of AD/ADRD; more than 85% of people surveyed can identify the disease and its symptoms. AD/ADRD is also one of the most feared health conditions, yet there are widespread and significant public misperceptions about diagnosis and clinical management. Misperceptions lead both to delayed diagnosis, and to people with the disease and their caregivers feeling isolated and stigmatized. Enhancing public awareness and engagement is essential because it forms the basis for advancing the other goals of the National Plan. A better understanding of AD/ADRD will help engage stakeholders who can work to address the challenges faced by people with the disease and their families. These stakeholders include a range of groups such as health care providers who care for people with AD/ADRD and their caregivers, employers whose employees request flexibility to care for a loved one with the disease, groups whose members are caregivers, and broader aging organizations. The strategies and actions under this Goal are designed to educate these and other groups about the disease.
Strategy 4.A: Educate the Public about Alzheimer’s Disease and Related Dementias
Greater public awareness of AD/ADRD can encourage families to seek assessment, reduce isolation and misunderstanding felt by caregivers, and help link people in need to accurate information, resources and services.
(UPDATED) Action 4.A.1: Enhance public outreach about Alzheimer’s disease and related dementias
Lead Agencies: ACL, NIA, CDC
Partners: multiple cross-agency and funded partners
Through its grant and resource center programs, ACL continues to build awareness of AD/ADRD. All ACL grantees include awareness and outreach in their programs. Numerous grantee programs include dementia-friendly community activities in their projects, partnering with established AD/ADRD stakeholders, as well as training volunteer educators of community organizations including, but not limited to, faith-based organizations, business leaders and grass roots volunteer organizations like Rotary clubs.
ACL’s NADRC website is an established hub for resources to support community outreach and education efforts. The website offers a broad range of resources to support the development and implementation of community-based AD/ADRD education programs.
For more information, see:
NIA operates the ADEAR Center, the primary Federal Government resource for information about AD/ADRD, research, and caregiving. The ADEAR Center educates the public about the latest research findings and provides evidence-based information online, in print and via a call center. Information about AD/ADRD, participation in clinical trials, and caregiving is freely available. NIA disseminates ADEAR’s resources through outreach in the research and care communities and through media and advocacy organizations, via weekly e-alerts to more than 50,000 subscribers, and social media outreach to more than 10,000 followers.
In 2020, NIA, working with other federal agencies, led efforts to update and enhance the Alzheimers.gov website. NIA launched this new portal to Federal Government information and resources in February 2021. The site features:
- Information about AD/ADRD.
- Tips and resources for caregivers and people living with dementia.
- Updates on Federal Government activities to address AD/ADRD.
- How to take part in clinical research and how to find studies.
- Resources for health care providers, community and public health workers, and researchers.
For more information, see:
CDC’s Alzheimer’s Disease Program publishes web features, a series of podcasts, weekly newsletters to more than 50,000 subscribers, and social media to more than 27,000 followers with the goal of increasing awareness and engagement by the public and its stakeholders about AD/ADRD. Web features for 2020-2021 include the following, most of which are also available in Spanish:
- Web Features
- Down Syndrome and Risk for Alzheimer’s (September 2021)
- Barriers to Equity in Alzheimer’s and Dementia Care
- Baby Boomers Who Are Caregivers Report Poor Health
- Healthy Body, Healthy Brain
- Loneliness and Social Isolation in Older Adults
- Podcasts (Aging and Health Matters Series)
- How Are You Feeling Right Now? Coping Strategies for Caregivers
- Social Isolation and Loneliness Among Older Adults and What You Can Do to Help
- Baby Boomers Who Are Caregivers Report Being in Poor Health
- Healthy Body, Healthy Brain
- Loneliness Puts Older Adults at Risk for Serious Medical Problems
- What About the Caregivers?
- The Importance of Physical Activity for Older Adults
For more information, see:
Web Features
- https://www.cdc.gov/aging/publications/features/barriers-to-equity-in-alzheimers-dementia-care/index.html
- https://www.cdc.gov/aging/publications/features/caregivers-baby-boomers-report-poor-health/index.html
- https://www.cdc.gov/aging/publications/features/healthy-body-brain.html
- https://www.cdc.gov/aging/publications/features/lonely-older-adults.html
Podcasts
- https://solesourcepodcast.buzzsprout.com/789140/8142833-ep55-caring-for-caregivers-with-the-cdc
- https://podcasts.apple.com/us/podcast/ep38-cdc-highlights-social-isolation-loneliness-among/id1508046828?i=1000501704755
- https://tools.cdc.gov/medialibrary/index.aspx#/media/id/412477
- https://tools.cdc.gov/medialibrary/index.aspx#/media/id/408892
- https://tools.cdc.gov/medialibrary/index.aspx#/media/id/407659
- https://tools.cdc.gov/medialibrary/index.aspx#/media/id/406088
- https://tools.cdc.gov/medialibrary/index.aspx#/media/id/405188
Additionally, CDC provided communication guidance and technical assistance to all its funded partners.
CDC has participated in the Did You Know? feature offered by CDC’s Center for State, Tribal, Local, and Territorial Support to promote prevention activities. Featured topics have included: baby boomers who are caregivers, brain health, memory loss, chronic conditions in relation to memory loss, and how dementia disproportionately effects minority populations and women.
CDC is taking steps to proactively address issues arising from COVID-19 affecting persons with dementia and their caregivers. CDC’s Alzheimer’s Disease Program developed a dedicated COVID-19 web page highlighting CDC’s COVID-19 guidance for older adults. Since its launch in March 2020, there have been more than 1.8 million pageviews through July 19, 2021. Content includes videos, fact sheets, infographics, and health equity considerations for racial and ethnic minority groups. Resources are available in multiple languages. Videos are also available in American Sign Language. The Alzheimer’s Disease Program disseminates a weekly newsletter to more than 67,000 subscribers. It is a primary channel for disseminating information about COVID-19 web updates and webinars. The Alzheimer’s Disease Program continues to provide older adult Subject Matter Experts to the COVID-19 pandemic response in CDC’s Emergency Operations Center.
For more information, see:
- https://www.cdc.gov/aging/index.html
- https://www.cdc.gov/aging/covid19-guidance.html
- https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/
CDC is also reaching and engaging the public and stakeholders through webinars, town halls, public messaging campaigns, and other outreach with partners. For example, CDC has worked closely with the Alzheimer’s Association, AARP, state and local health departments, state offices of aging, and national clinician groups to share information on how older adults, persons with dementia, and caregivers can protect their communities from COVID-19. CDC is also collaborating on several projects to reduce social isolation and maintain mental health among older adults. CDC works closely with the CDC Foundation and other partners to ensure that disproportionately impacted communities receive the resources and technical assistance necessary to provide COVID-19 related services to older adults.
(ONGOING) Action 4.A.2: Facilitate translation of data and surveillance to inform the public
Lead Agency: CDC
Partners: Private partners
Since 2019, CDC has translated the national caregiving and SCD infographics into Spanish and develop corresponding infographics for Black, AI/AN, AAPI, Hispanic, and LGBT individuals, as well as women, men, residents of rural areas, and veterans. These infographics can be used to educate the public and aid in making decisions on how to allocate resources and funding.
For more information, see:
Additionally, CDC developed infographics co-branded with the Alzheimer’s Association and IHS. These resources are marketed on the Alzheimer’s Association website and distributed to a national network of state Alzheimer’s Association offices, public health professionals, and decision makers.
The State of Aging and Health in America: Data Brief Series are topic-specific documents focusing on public health issues related to older adults developed by CDC and the National Association of Chronic Disease Directors (NACDD). These briefs provide public health professionals with the most recent data available on health and aging-related conditions, including the importance of brain health, the management of chronic conditions, and caregiving burdens, to help identify needs and mitigate the future effects of a growing older population. The briefs also provide data by important breakdowns such as by state, age, gender, and ethnicity which can be useful for states and other stakeholders in making informed decisions and policies related to these issues.
For more information, see:
CDC also supported the Alzheimer’s Association to develop a Needs Assessment Toolkit that serves as Guidance and Resources for State Public Health Agencies on Comprehensive Needs Assessments Related to Alzheimer’s and Other Dementias. This document aims to increase the use of information and insights to appropriately respond to the growing public health burden associated with AD/ADRD through comprehensive needs assessments. These are at the core of a state’s ability to effectively use information to develop, implement, and maintain state plans that are focused either exclusively on AD/ADRD, or more broadly on the incorporation of cognitive health and impairment into other state public health plans. Public health agencies have a high-level of expertise related to developing and conducting needs assessments. This toolkit has been developed to help states leverage their expertise in conducting needs assessments so as to enhance their ability to gather and use information specifically related to AD/ADRD.
Also see Action 1.E.3 for information on resources developed using BRFSS data.
Strategy 4.B: Work with State, Tribal, and Local Governments to Improve Coordination and Identify Model Initiatives to Advance Alzheimer’s Disease and Related Dementias Awareness and Readiness across the Government
State, Tribal, and local governments are working to help address challenges faced by people with AD/ADRD and their caregivers. Nineteen states and a handful of local entities have published plans to address AD/ADRD that cover many of the same issues as the National Plan. Leveraging the available resources and programs across these levels of government will aid in the success of these efforts.
(ONGOING) Action 4.B.1: Continue to convene federal partners
Lead Agency: ASPE
Partners: CDC, NIH/NIA, ACL, CMS, HRSA, AHRQ, IHS, SAMHSA, OASH, VA, NSF, DoD
The Interagency Group on Alzheimer’s Disease and Related Dementias, convened on an ongoing basis since April 2011, provides a forum for discussion of AD/ADRD efforts across federal departments and agencies. Participants in this group have gained a better understanding of the roles and responsibilities of other departments and agencies for addressing AD/ADRD. Together, the group has identified existing resources and new opportunities for collaboration, best practices, and initiatives. HHS will continue to convene federal partners to collaborate on AD/ADRD. The group will share research findings, innovative or best practices, and information about new or upcoming initiatives.
(UPDATED) Action 4.B.2: Build upon lessons learned to improve the dementia-capacity of state and local service systems
Lead Agencies: ACL, CDC
Partners: CMS
HHS will improve the dementia-capability of state and community service systems through the ACL’s ADPI and NADRC. ACL and the NADRC have developed the Dementia Capability Assessment Tool designed to measure the dementia-capability of the LTSS in various organizations and measure improvement over time.
ADPI grantees, partners and other collaborators will work in peer-led groups on specific activities to make state and local-level improvements related to dementia-capability. The peer-led groups will develop practical tools to promote the adoption of dementia-capable practices at the state and local levels. HHS will help states and communities meet the needs of people with AD/ADRD through an expanded Dementia Capability Toolkit and other, related resources. Additional materials will result from similar program activities.
For more information, see:
CDC BOLD Program awardees are funded to create and maintain jurisdiction-wide coalitions to collaborate on setting AD/ADRD priorities informed by data for their area. The state and local jurisdictions of Colorado, Georgia, Los Angeles County, Maine, Mississippi, Nevada, Iowa, Oklahoma, Vermont, Wisconsin and North Carolina have all created statewide AD/ADRD coalitions to guide strategic planning for people with AD/ADRD and their caregivers. The BOLD program awardees are all working on updating their statewide AD/ADRD plans to include a minimum of four actions from the State and Local Public Health Partnership to Address Dementia: The 2018-2023 Road Map.
(ONGOING) Action 4.B.3: Get Tribal input on Alzheimer’s disease and related dementias and support improved coordination between Indian Health Service, Tribal, and Urban Indian Health programs and the Tribal aging network
Lead Agencies: IHS, ACL
Partners: ASPE, VA
HHS will solicit input from Tribal leaders on the impact of AD/ADRD on Indian Country during the annual Tribal Consultation process and through broader meetings and convenings. HHS will use these opportunities to convene leaders and solicit input on the needs related to recognition, diagnosis, and support for individuals with dementia and their families.
The Alzheimer’s Association, in collaboration with CDC, has started meetings with the United South and Eastern tribes and with the Northwest Portland Area Indian Health Board. There has been increased Tribal representation on the CDC HBI Road Map for Indian Country work.
(ONGOING) Action 4.B.4: Develop and update a public health road map for assisting state, Tribal, and local health departments in prioritizing actions
Lead Agency: CDC
CDC supported the Alzheimer’s Association to co-develop the third in a series of HBI Road Maps to advance cognitive health as an integral component of public health, the HBI State and Local Public Health Partnerships to Address Dementia. The 2018-2023 Road Map outlines how state and local public health agencies and their partners can continue to promote cognitive health, address cognitive impairment for people living in the community, and help meet the needs of caregivers. Twenty-five specific actions are proposed in four traditional domains of public health: educate and empower, develop policies and mobilize partnerships, assure a competent workforce, and monitor and evaluate.
In collaboration with the Alzheimer’s Association and numerous partners, the Road Map for Indian Country was released in 2019 and disseminated to multiple stakeholders and Tribal leaders. This Road Map has been designed specifically for public health systems serving AI/AN and Native Hawaiians.
For more information, see:
- https://www.cdc.gov/aging/healthybrain/Indian-Country-resources.html
- https://www.cdc.gov/aging/healthybrain/Indian-country-roadmap.html
- https://www.cdc.gov/aging/healthybrain/roadmap.htm
Strategy 4.C: Coordinate United States Efforts with Those of the Global Community
Many nations have developed dementia plans of their own that involve improved care and supports for people with AD/ADRD and their caregivers, as well as enhanced research and public awareness. In implementing the Actions in this Plan, HHS and its federal partners will coordinate with global partners to enhance these plans, avoid duplication of effort, and optimize existing resources.
(ONGOING) Action 4.C.1: Work with global partners to enhance collaboration
Lead Agencies: ASPE, NIA
The United States participated in the World Health Organization’s Global Dementia Observatory (GDO) in 2019. The GDO is an information exchange platform that collects information from countries on dementia policy, service delivery, and information and research. As of August 2019, 21 other countries had submitted information to the GDO.
See Action 1.D.2 for information on the HRS HCAP initiative, an innovative approach to assessing trends in cognitive function and aging in the United States and worldwide.
HCAP 2021, a follow-up to the original, is now being planned. Researchers will readminister the same in-home cognitive assessment and seek an informant report from all surviving members of the original HCAP sample and from a new random sample of those age 65-68 in 2021. HCAP 2021 will provide extensive new data to better assess trajectories of cognitive decline. These data afford an unprecedented opportunity to more clearly describe trends in the incidence and prevalence of dementia around the world.
See Action 1.D.2 for information on administration of HCAP in other developed and developing countries. In most of these studies, important biomarker data, including DNA for genotyping and future sequencing, is also being collected; genotype information is already available for the United States, England, and Mexico studies.
See Action 1.D.2 for information on the HCAP network, which aims to develop international data resources for the study of AD/ADRD that will expand research opportunities to exploit cross-country variation in key life-course factors that likely affect cognitive function and the risk for AD/ADRD.
For more information, see:
- https://www.nia.nih.gov/research/blog/2019/05/healthy-cognitive-aging-project-major-data-resource-cognitive-epidemiology
- https://hrsdata.isr.umich.edu/data-products/2016-harmonized-cognitive-assessment-protocol-hcap?_ga=2.118334926.654419972.1601312536-181621991.1601312536
- https://hcap.isr.umich.edu/
- https://g2aging.org/
NIA also supports an international team of researchers that has made more progress in explaining the genetic component of AD/ADRD. Their analysis, involving data from more than 35,000 individuals with LOAD, has identified variants in five new genes that put people at greater risk of AD/ADRD. It also points to molecular pathways involved in AD/ADRD as possible avenues for prevention and offers further confirmation of 20 other genes that had been implicated previously in AD/ADRD. The results of this largest-ever genomic study of AD/ADRD suggests key roles for genes involved in the processing of beta-amyloid peptides, which form plaques in the brain recognized as an important early indicator of AD/ADRD. They also offer the first evidence for a genetic link to proteins that bind tau, the protein responsible for telltale tangles in the AD/ADRD brain that track closely with a person’s cognitive decline. The new findings are the latest from the International Genomics of Alzheimer’s Project consortium. The effort, spanning four consortia focused on AD/ADRD in the United States and Europe, was launched in 2011 with the aim of discovering and mapping all the genes that contribute to AD/ADRD.
For more information, see:
Goal 5: Improve Data to Track Progress
The Federal Government is committed to better understanding AD/ADRD and its impact on people with dementia, families, the health and long-term care systems, and society as a whole. Data and surveillance efforts are paramount to tracking the burden of AD/ADRD on individual and population health and will be used to both identify and monitor trends in risk factors associated with AD/ADRD and assist with understanding health disparities among populations such as racial and ethnic minorities, low income populations, rural residents, and sexual and gender minorities. HHS will make efforts to expand and enhance data infrastructure and make data easily accessible to federal agencies and other researchers. This data infrastructure will help HHS in its multi-level monitoring and evaluation of progress on the National Plan.
Strategy 5.A: Enhance the Federal Government’s Ability to Track Progress
The Federal Government needs improved data on people with AD/ADRD, their caregivers, and the care and supports that they use to address policy questions and plan and evaluate new initiatives. HHS and its partners will identify the policy questions that cannot be answered with existing data, as well as questions likely to arise in the future. These questions will provide a mechanism for identifying gaps, challenges, and changes or additions to data collection.
(ONGOING) Action 5.A.1: Identify needed changes or additions to data
Lead Agency: ASPE
Partners: CMS, CDC, NIA, ACL, VA, IHS
HHS will work with federal partners and researchers to identify the data and data infrastructure needed to address new policy issues. These changes or additions may include new or improved measures, new data collection efforts, or links between existing datasets.
(ONGOING) Action 5.A.2: Make needed improvements to data
Lead Agency: ASPE
Partners: CDC/NCHS, NIA
HHS will address the identified data needs or possible improvements and develop questions to be fielded for data collection. These questions may be added to existing studies, be part of supplements to existing studies, or form the basis of a new study.
(UPDATED) Action 5.A.3: Summarize data on cognitive impairment across states
Lead Agency: CDC
CDC continues to summarize and provide infographics from data on cognitive impairment across states. See Action 4.A.2 for a description of the State of Aging and Health in America: Data Brief Series, developed by CDC in collaboration with NACDD.
(ONGOING) Action 5.A.4: Summarize existing data on people with Alzheimer’s disease and related dementias and their caregivers
Lead Agency: CDC, ODPHP
Partners: ASPE, NCHS, NIA, ACL
CDC, NIA, and ACL provided new data benchmarks and goals related to AD/ADRD through Healthy People 2020 and Healthy People 2030. During the Healthy People 2020 close-out, more recent data was provided for “DIA-1: Increase the proportion of adults aged 65 years and older with diagnosed Alzheimer’s disease and other dementias, or their caregiver, who are aware of the diagnosis” and “DIA-2: Reduce the proportion of preventable hospitalizations in adults aged 65 years and older with diagnosed Alzheimer’s disease and other dementias”. For Healthy People 2030, the dementia workgroup successfully retained DIA-1 and DIA-2 and added a third core objective “DIA-3: Increase the proportion of adults with SCD who have discussed their confusion or memory loss with a health care professional”. These three objectives each set new and ambitious targets to be achieved during the next decade to improve health and quality of life for people with dementia, including AD/ADRD.
For more information, see:
Also see Action 4.A.2 for a description of the State of Aging and Health in America: Data Brief Series developed by CDC in collaboration with NACDD, and Action 1.E.3 for update on the caregiving and SCD infographics.
(ONGOING) Action 5.A.5: Provide analysis of Behavioral Risk Factor Surveillance System data on Alzheimer’s disease and related dementias and their caregivers in user-friendly formats
Lead Agency: CDC
Partner: NACDD
CDC partnered with NACDD to create a series of data briefs addressing topic-specific public health issues related to older adults. These briefs provide public health professionals with the most recent data available on health and aging-related conditions including the importance of brain health, the management of chronic conditions, and caregiving burdens so as to help identify needs and mitigate the future effects of a growing population of older adults. The briefs also provide data by important breakdowns such as by state, age, gender, and ethnicity which can be useful for states and other stakeholders in making informed decisions and policies related to these issues.
For more information, see:
Strategy 5.B: Monitor Progress on the National Plan
The National Plan is intended to be a road map for accomplishing its five goals. It is a document that is designed to be updated regularly. HHS is committed to tracking progress and incorporating findings into an updated National Plan.
(ONGOING) Action 5.B.1: Track National Plan progress
Lead Agency: ASPE
HHS will monitor progress to determine whether actions are being completed as stated in the National Plan, and the extent to which implemented actions contribute to the desired outcomes and changes associated with each strategy. HHS and its federal partners will identify challenges to the successful completion of Strategies and Actions, and make recommendations for how they can be addressed. For each strategy, HHS will monitor available population-based data, such as the NHATS, Medicare Current Beneficiary Survey, or the BRFSS to assess the extent to which progress is being made. HHS will use data from both the public and private sectors, as appropriate, to track progress on the National Plan. Additionally, HHS will work to incorporate measures related to AD/ADRD into other surveillance efforts to monitor population health, such as Healthy People 2020 and Healthy People 2030 which incorporate objectives related to AD/ADRD.
For each Action, HHS will track implementation to determine whether actions are completed in a timely and successful manner. Progress on each of these actions will be reported to the Advisory Council.
(ONGOING) Action 5.B.2: Update the National Plan annually
Lead Agency: ASPE
Tracking progress will help HHS and the Advisory Council monitor progress towards the goals of the National Plan and make recommendations for priority actions and updates to the National Plan. HHS will incorporate its findings and the recommendations of the Advisory Council to update the National Plan on an annual basis.
(ONGOING) Action 5.B.3: Identify key indicators of progress on the National Plan
Lead Agency: ASPE
ASPE convened federal partners to identify key indicators of progress on the National Plan. These indicators should be meaningful for the policy makers, program staff, as well as the public, and enhance our understanding of the impact of the activities described within the framework of the National Plan. Indicators may be available from federal program data or other sources. As a next step, ASPE will host a discussion of this work with members of the NAPA Advisory Council.
Goal 6: Accelerate Action to Promote Healthy Aging and Reduce Risk Factors for Alzheimer’s Disease and Related Dementias
While there is currently insufficient evidence that dementia can be prevented, a growing body of research has identified modifiable risk factors for AD/ADRD, and suggests that strategies to reduce the burden of these risk factors may delay onset or slow progression of AD/ADRD and its symptoms. The relationship between hypertension management and cognitive health is among the most robust studied; other activities to address other potential risk factors for AD/ADRD include cognitive training and engaging in physical activity, among others. These same activities to preserve cognitive health are also conducive to healthy aging overall. Evidence on the relationship between modifiable risk factors and the incidence of AD/ADRD is evolving, as is research on the effectiveness of interventions to reduce risk.
Under this Goal, the Federal Government will accelerate research on risk factors for AD/ADRD, and strengthen the infrastructure that is necessary to rapidly translate and disseminate information about risk factors, interventions to reduce the burden of risk factors, and related health promotion activities to health care providers, community-based providers, and public health networks.
The burden of risk factors for AD/ADRD is disproportionately high among certain racial and ethnic groups (e.g., Black, Hispanic, and AI/AN populations), and among adults with lower SES. These disparities in the prevalence of risk factors -- which are grounded in generations of structural racism and inequality in health care -- contribute to disparities in the incidence of AD/ADRD that are further amplified by disparities in AD/ADRD diagnosis, treatment, and access to care and resources. It is therefore of critical importance that research, interventions, and infrastructure to address modifiable risk factors for AD/ADRD are culturally responsive and grounded in improving equity by addressing the social determinants of health (SDOH). Accordingly, future efforts to reduce the burden of risk factors for AD/ADRD will focus on understanding not only what actions individuals can take to reduce their risks, but also what community and system-level investments are needed to facilitate risk reduction and support healthy aging.
Strategy 6.A: Identify Research Priorities and Expand Research on Risk Factors for Alzheimer’s Disease and Related Dementias
While NIH has supported dementia risk reduction research for decades, identifying the priorities and milestones to achieve Goal 6 requires increased attention by the research community. Much of the current evidence on modifiable risk factors is low to moderate quality, so more research is needed to better understand the relationship between potential risk factors and AD/ADRD. The actions below will identify the priorities, establish milestones, and ensure that appropriate stakeholders are involved in the planning process aimed at identifying and addressing modifiable risk factors. Through this work, NIH and partner agencies will develop research priorities and a plan for implementing each phase of research in a coordinated manner.
(NEW) Action 6.A.1: Enhance the focus on risk reduction in existing research summits
Lead Agencies: NIA/NINDS/NIH
Under Action 1.A.1, NIH convenes a series of annual research summits to address a wide range of critical research issues in AD/ADRD, including basic, translational, and clinical research, as well as research on care and LTSS. Gaps and opportunities identified by participants providing individual input at these summits are used to inform research planning at the NIH. In order to advance research on risk reduction, beginning with the next Alzheimer’s Disease Research Summit in 2023, NIA and NINDS will ensure that risk reduction is integrated into the summits. As NIH develops plans for future summits, it will continue to engage a diverse mix of investigators, representatives from non-governmental organizations, industry, people living with dementia and their caregivers, and other communities in both organizing the meeting and in leading the individual meeting sessions. Consideration will be given to gender diversity, as well as ensuring diverse representation from underrepresented racial and ethnic groups, individuals with disabilities, and individuals from socially and economically disadvantaged backgrounds.
(ONGOING) Action 6.A.2: Monitor and improve access to public health surveillance data to identify risk factors and establish research priorities
Lead Agency: CDC
CDC monitors data from the Cognitive Decline Module of the BRFSS and the Cognitive Performance and SCD module to the National Health and Nutrition Examination Survey (NHANES). In 2022, CDC will produce and disseminate a scientific paper examining the association between risk factors and SCD using the BRFSS. CDC will also be examining risk factors using the NHANES data.
To improve access to the monitoring capabilities of the BRFSS data, in early 2021, CDC released a revised Technical Assistance Document for both the Caregiving and Cognitive Decline Modules designed to provide guidance for BRFSS coordinators and researchers who would like to conduct analyses of the data collected through the 2015-2020 BRFSS Caregiver or Cognitive Decline Optional Modules. These documents provide basic computer code for analyzing the data with a goal to enable consistency in analytic methods and results reported. The BRFSS data is publicly-available for users.
CDC has made data from the BRFSS Caregiver and Cognitive Decline Modules available in user-friendly formats, to facilitate broader use of these data. These include a searchable data portal, data briefs, and infographics with national estimates, by state, sex, rural status, veteran status, and race/ethnicity.
For more information, see:
- https://www.cdc.gov/aging/healthybrain/brfss-faq-cognitive.htm
- https://www.cdc.gov/aging/publications/BRFSS-caregiver-brief-508.pdf
- https://www.cdc.gov/aging/publications/briefs.htm
- https://www.cdc.gov/aging/data/BRFSS-statistical-brief-cognitive-decline-508.pdf
- https://www.cdc.gov/aging/data/index.htm
- https://www.cdc.gov/aging/agingdata/index.html
- https://www.cdc.gov/brfss/
- https://www.cdc.gov/nchs/nhanes/
(NEW) Action 6.A.3: Expand and diversify clinical research studies on promising interventions to reduce individual and community-level risk
Lead Agencies: NIA/NINDS/NIH
NIH is funding a wide range of clinical research studies and trials designed to better understand the complex interplay of risk and protective factors for AD/ADRD, and to test interventions to reduce the burden of those risk factors and ultimately decrease the incidence of disease downstream and promote cognitive health. Both NIA and NINDS will continue to monitor emerging evidence in the field, including newly identified risk and protective factors, and expand future research investments in the most promising areas.
(NEW) Action 6.A.4: Enhance research to better understand the varying levels of or types of dementia risk across demographic groups
Lead Agencies: NIA/NINDS/NIH
Emerging research suggests that differences in the risks of AD/ADRD reflects differences in both modifiable (e.g., physical activity and education) and non-modifiable factors (e.g., genetics). NIH’s strategic planning efforts around AD/ADRD reflect a prioritization of issues related to the racial and ethnic disparities of these conditions. For example, NIA has developed a National Strategy to improve recruitment of racial and ethnic minorities in its research, both intramural and external. Both NIA and NINDS will continue to invest in these areas of research at the basic, translational, clinical, and epidemiological levels to understand these risk factors and the impact they have on disparities in AD/ADRD between these populations.
Several new and ongoing clinical studies are seeking to determine risk profiles for AD/ADRD, especially in the area of vascular risk factors. For approximately 20 years, NINDS has supported the Reasons for Geographic and Racial Differences in Stroke (REGARDS), a longitudinal prospective study of stroke risk in racial and ethnic minorities as well as low SES and rural populations. NINDS and NIA have expanded the study’s goals to now include understanding disparities in the risk for dementia and cognitive decline as well as stroke. In 2020, the NINDS-funded Blood Pressure-Cognition Study reported the results of a meta-analysis of five large NIH cohort studies (including REGARDS described above), pooling together data from nearly 20,000 participants. The study found that high blood pressure is linked to faster rates of cognitive decline among adults, suggesting that controlling high blood pressure, especially in midlife, may be an effective approach to preventing cognitive decline and dementia. In addition, the analysis confirmed previous findings from other studies that Black Americans experienced cognitive decline earlier than White Americans, and it also showed that uncontrolled high blood pressure in Black individuals appears to explain later disparities in rates of dementia.
For more information, see:
- https://reporter.nih.gov/project-details/10118228
- https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-19-012.html
- https://pubmed.ncbi.nlm.nih.gov/32282019/
- https://projectreporter.nih.gov/project_info_description.cfm?aid=9918026&icde=51396638&ddparam=&ddvalue=&ddsub=&cr=5&csb=default&cs=ASC&pball
(ONGOING) Action 6.A.5: Expand research on traumatic brain injury as a risk factor for neurodegeneration
Lead Agencies: DOD, NINDS/NIH
Partner: VA
Several research studies have suggested a connection between TBI and later incidence of dementia, but additional investigation is needed to confirm and better understand the mechanism involved. DOD, NINDS, and VA are supporting further research to understand the brain changes resulting from TBI and potential relationships with subsequent neurodegeneration. A key goal of this research is to understand whether protective factors or interventions can improve the course and/or severity of neurodegenerative outcomes.
The VA Office of Research and Development continues to support research projects to advance our knowledge of the long-term impacts of TBI on brain health, employing a four-prong approach towards understanding and treating TBI-related AD/ADRD. These approaches include: Analyses of VA EHRs and other large datasets, supporting longitudinal cohort studies, developing medically actionable biomarkers, and conducting early-stage clinical trials on therapeutics.
(NEW) Action 6.A.6: Expand research on the impact of emerging potential risk factors such as COVID-19
Lead Agencies: NINDS/NIA/NIH
Over the past 2 years it has become evident that many diverse individuals who have contracted SARS-CoV-2 experience either a greatly prolonged period of illness (i.e., “long COVID”) or longer-term post-acute sequelae following acute COVID-19 related illness (i.e., PASC) that include but are not limited to neuropathological insult and significant cognitive changes. To investigate these further, NIH has recently launched RECOVER (Researching COVID to Enhance Recovery), a research initiative designed to understand, prevent, and treat the post-acute effects of SARS-CoV-2. NINDS and NIA will continue to participate actively in this critical effort and will consider gaps emerging from PASC findings for future investments at the institute level.
For more information, see:
(UPDATED) Action 6.A.7: Continue clinical trials on the most promising health promotion interventions
Lead Agency: NIA
Partners: VA
See Action 1.B.5 for updates regarding the ACTC and ongoing clinical trials supported by NIA. Over 110 of the approximately 270 active trials of interventions to enhance cognitive health in older adults and to prevent, treat, or manage AD/ADRD investigate non-pharmacological interventions, including testing lifestyle factors such as diet and exercise. NIA has also released FOAs specifically focused on clinical trials for AD/ADRD.
For more information, see:
- https://grants.nih.gov/grants/guide/pa-files/PAR-18-513.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-20-029.html
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-22-016.html
- https://www.nia.nih.gov/news/new-nih-consortium-award-enhance-clinical-trials-alzheimers-disease-related-dementias
- https://www.nia.nih.gov/research/ongoing-AD-trials
In 2019, two major reports were published from the SPRINT-MIND clinical research study, which was the first randomized control trial of its size and scope to examine a modifiable risk factor for dementia. The study established that intensively lowering blood pressure in participants aged 50 and above decreased their risk for MCI by approximately 20% and reduced progression of white-matter hyperintensities, which are associated with vascular damage in the brain. Findings from a related clinical trial called INtensive versus Standard Ambulatory Blood Pressure Lowering to Prevent Functional DeclINe In The ElderlY (INFINITY) were consistent with the SPRINT-MIND results. The INFINITY trial indicated that, after 3 years of treatment, intensive lowering of blood pressure slowed white-matter disease in adults age 75 and older with high blood pressure.
For more information, see:
- https://pubmed.ncbi.nlm.nih.gov/30688979/
- https://pubmed.ncbi.nlm.nih.gov/31408137/
- https://www.nih.gov/news-events/news-releases/intensive-blood-pressure-control-may-slow-age-related-brain-damage
- https://www.nia.nih.gov/news/intensive-blood-pressure-control-slowed-white-matter-disease-adults-age-75-and-older
NIA funds many clinical trials on health-related behaviors and dementia, including combinations of healthy behaviors. Because these behaviors may need to start decades before disease onset, understanding the factors that support long-term adherence to lifestyle change will be critical. In early 2021, NIA released new funding opportunities to support research, including behavior change clinical trials, on the psychology of motivation, value-based decision making, and social support. The hope is that findings from this line of research will help investigators develop ways to help people adopt and sustain healthy behaviors over many years.
For more information, see:
- https://www.nia.nih.gov/research/ongoing-AD-trials#section3
- https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-22-016.html
- https://grants.nih.gov/grants/guide/pa-files/PAR-21-207.html
NIA’s HCAP Network aims to develop international data resources for the study of AD/ADRD that will expand research on key life-course factors that are thought to affect cognitive function and increase risk for AD/ADRD. This support for global research provides a broader database regarding health-related behaviors, diets, and environmental factors, expanding insight on potential risk and protective factors of AD/ADRD. See Action 1.D.2 for more detail and resources.
Since 2020, VA has been one of the recruitment networks for the NIA-funded PREVENTABLE trial, which aims to determine whether statin can prevent dementia and disability in addition to heart disease and other cardiovascular-related deaths. The VA CSP Pharmacy Coordinating Center serves as the central pharmacy for the trial to distribute medications to study participants. VA continues to support clinical trials of interventions to reduce risks for developing AD or alleviating the symptoms.
Strategy 6.B: Facilitate Translation of Risk Reduction Research Findings into Clinical Practice
As understanding of potential modifiable risk factors emerges, the Federal Government will quickly disseminate information and educate health care providers about risk factors and interventions to reduce their burden, so that when appropriate such measures can be considered in clinical settings through informed and shared decision making. Dissemination of research findings to clinical settings will also provide individuals with information about what may help in delaying the onset and/or slowing the progression of AD/ADRD, and resources available to support them.
(NEW) Action 6.B.1: Educate the health care workforce about risk reduction
Lead Agencies: HRSA, CDC
It is essential for the health care workforce to understand the risk factors for dementia in order to promote risk reduction among adults. HRSA will use its network of GWEPs to develop and disseminate curricula to train the health care workforce in using a “whole-person” approach that encompasses all of patient’s needs to address individuals’ brain and behavioral health. HRSA will require geriatrics workforce development programs to include training on the AWV.
To increase providers awareness of brain health, CDC supported the ACPM to develop a Brain Health Continuing Education Course and Resource Website to increase physician and health care professionals’ awareness of brain health as a serious health condition. In 2021, the ACPM and CDC published an article, Cognitive Decline and Dementia Risk Reduction: Promoting Healthy Lifestyles and Blood Pressure Control, which describes how healthy choices can reduce the risk of cognitive decline and the importance of treating and managing hypertension in midlife. These brain health resources have been disseminated through the CDC’s weekly newsletter and ACPM mechanisms.
For more information, see:
- https://www.acpm.org/education-events/continuing-medical-education/2019/brain-health-benefits-of-blood-pressure-management/?utm_source=Website&utm_medium=CDC&utm_campaign=Brain%20Health
- https://www.acpm.org/initiatives/brain-health/brain-health-resources/
- https://www.sciencedirect.com/science/article/pii/S0749379721002002?via%3Dihub
(NEW) Action 6.B.2: Increase access to hearing aids for individuals with hearing loss
Lead Agency: FDA
Hearing loss has been identified as a risk factor for AD/ADRD, and recent research has demonstrated that hearing aid use is associated with reduced dementia risk. Hearing aids are often expensive, making them inaccessible to many individuals who could benefit.
FDA recently issued a Proposed Rule to establish a new regulatory category for over-the-counter (OTC) hearing aids and to make related amendments to update the regulatory framework for hearing aids. Such action would entail defining OTC hearing aids and their requirements, and amending existing rules to be consistent with the new category. The Proposed Rule aims to foster innovation in hearing aid technology. It would also improve access to hearing aids, as OTC options would likely be easier to obtain and less expensive.
For more information see:
- https://www.fda.gov/news-events/press-announcements/fda-issues-landmark-proposal-improve-access-hearing-aid-technology-millions-americans
- https://www.federalregister.gov/documents/2021/10/20/2021-22473/medical-devices-ear-nose-and-throat-devices-establishing-over-the-counter-hearing-aids
- https://pubmed.ncbi.nlm.nih.gov/33614893/
(ONGOING) Action 6.B.3: Disseminate research on co-occurring chronic conditions and dementias
Lead Agency: CDC
CDC has partnered with NACDD to develop a series of customizable Rack Cards for distribution at public health and other medical clinics and other appropriate areas, including health fairs or other health promotional events. These Rack Cards, which are in both Spanish and English, are designed to educate patients about risk reduction practices related to AD/ADRD, including the importance of blood pressure control, physical activity, healthy diet, blood sugar management, and smoking cessation. The Rack Cards are being adapted by state health departments with technical assistance from CDC and NACDD. These risk reduction messages can then be integrated alongside existing health promotion messaging efforts among states and other partners.
In collaboration with the Alzheimer’s Association, Association of State and Territorial Health Officials (ASTHO), and IA2, developed a series of four customizable templates and two instruction guides for Healthy Heart, Healthy Brain for use by health care providers and public health professionals. The templates include steps patients can take to promote heart, brain, and overall health.
For more information, see:
- https://www.cdc.gov/aging/partnership/nacdd-partner-resources/index.html
- https://www.cdc.gov/aging/partnership/partner-resources/index.html
(ONGOING) Action 6.B.4: Encourage treatment of co-occurring behavioral health conditions
Lead Agency: SAMHSA
Partners: CMS, HRSA, ACL
Behavioral health conditions, including depression, other mental illnesses, and substance use disorders (SUD), are risk factors for AD/ADRD.[17, 18, 19] Approaches to treatment for depression can be found in Substance Abuse and Mental Health Services Administration’s (SAMHSA’s) Treatment of Depression in Older Adults Evidence-Based Practices (EBP) KIT. The kit offers information about an array of EBP for treatment and services to improve outcomes for older adults experiencing depression, including dysthymia. It considers planning, implementation, and maintenance. Treatment approaches for older adults with dementia or other cognitive impairments are included in the case examples.
If a person is in an early stage of AD/ADRD, psychosocial therapies for serious mental illness (SMI) may still be effective, although the interventions may not be appropriate if a person is in a more advanced stage of dementia. SAMHSA’s guide for practitioners on Psychosocial Interventions for Older Adults With SMI provides considerations and strategies for interdisciplinary teams, peer specialists, clinicians, registered nurses, behavioral health organizations, and policy makers in understanding, selecting, and implementing evidence-based interventions that support older adults with SMI. In addition, SAMHSA, CMS, HRSA, and ACL collaborated to publish Guidance on Inappropriate Use of Antipsychotics: Older Adults and People with IDD in Community Settings. The Guidance reviews non-pharmacologic behavioral approaches and strategies to avoid and reduce prescribing of antipsychotics whenever possible for older adults with dementia and people with IDD.
For more information, see:
- https://store.samhsa.gov/product/Treatment-Depression-Older-Adults-Evidence-Based-Practices-EBP-Kit/SMA11-4631
- https://store.samhsa.gov/product/Guidance-on-Inappropriate-Use-of-Antipsychotics-Older-Adults-and-People-with-Intellectual-and-Developmental-Disabilities-in-Community-Settings/PEP19-INAPPUSE-BR
- https://store.samhsa.gov/product/psychosocial-interventions-older-adults-serious-mental-illness/PEP21-06-05-001?referer=from_search_result
Some individuals with AD/ADRD may have SUD, which should also continue to be treated. As we age, the body’s ability to process alcohol and other substances becomes less effective; cognitive impairment can also alter the impacts of alcohol and other substances. Effective treatment approaches can be found in Treatment Improvement Protocol (TIP) 26: Treating SUD in Older Adults. TIP 26 is designed to help providers better understand how to identify, manage, and prevent SUD in older adults. The TIP describes the unique ways in which the signs and symptoms of SUD may manifest in older adults, drug and alcohol use disorder screening tools, assessments, and treatments tailored for older adults’ needs, the interaction between SUDs and cognitive impairment, and strategies to help providers improve their older clients' social functioning and overall wellness. A related resource from SAMHSA is the toolkit, Get Connected: Linking Older Adults with Resources on Medication, Alcohol, and Mental Health. The toolkit is designed for organizations that provide services to older adults and offers information and materials to help understanding the issues associated with substance misuse and mental illness in older adults.
SAMHSA partnered with HRSA to develop Growing Older: Providing Integrated Care for an Aging Population. The report is designed for clinicians and explains approaches to providing integrated care to older adults living with SUD and mental illness. It highlights the importance of assessing patients for cognitive deficits and adapting behavioral interventions to help improve treatment outcomes. The report also stresses the importance of including family caregivers, when possible, in the diagnostic and treatment process.
For more information, see:
- https://store.samhsa.gov/product/treatment-improvement-protocol-tip-26-treating-substance-use-disorder-in-older-adults/PEP20-02-01-011
- https://store.samhsa.gov/product/Get-Connected-Linking-Older-Adults-with-Resources-on-Medication-Alcohol-and-Mental-Health-2019-Edition/SMA03-3824
- https://store.samhsa.gov/product/Growing-Older-Providing-Integrated-Care-for-An-Aging-Population/SMA16-4982
Strategy 6.C: Accelerate Public Health Action to Address the Risk Factors for Alzheimer’s Disease and Related Dementias
While clinical health focuses on the individual, public health focuses on a population with the aim of protecting and promoting healthy people and communities. Developing the public health infrastructure and educating the public health workforce about AD/ADRD risk factors can ensure that as high-quality research emerges, public health systems can more rapidly advance interventions and investments targeting communities with greatest need to achieve more equitable outcomes.
(NEW) Action 6.C.1: Convene summit to establish public health priorities for reducing Alzheimer’s disease and related dementias risk factors
Lead Agency: CDC
To establish and update priorities and milestones, CDC is convening a National Summit on Risk Reduction in 2022. This Summit will include academic and public health partners gathering, as well as public health practitioners, state, local, and Tribal public health officials, ASTHO, and National Association of County and City Health Officials (NACCHO). This Summit will yield a list of public health strategies determined to be most appropriate for translation based on the state of the latest science, to be implemented by state, local, and Tribal public health entities. A second Summit will be held in 2024.
(ONGOING) Action 6.C.2: Accelerate dissemination of information on risk reduction to public health entities
Lead Agency: CDC, ODPHP
Partners: NACDD, ASTHO, NACCHO
CDC is partnering with ASTHO to produce a series of products to support public health agencies in identifying priorities, areas of synergy within existing or upcoming jurisdictional plans, and opportunities for integrating cognitive health into public health efforts as guided by the HBI Road Maps. Products designed to facilitate implementation of the HBI Road Maps include a series of HBI Action Institutes, health communication materials for AI/AN communities, healthy aging, and a series of recorded webinars to promote the importance of public health in addressing brain health. The communication materials for AI/AN communities are now customizable.
CDC supported the NACDD to develop brain health messaging that could be integrated into existing public health messaging. The initial Rack Cards were released in 2020 for four key risk factors related to brain health, in 2021 these are now customizable, and in 2022 NACDD is working to integrate these messages within state and local public health departments.
In 2021, in collaboration with NACCHO, CDC awarded the inaugural cohort of the HBI Road Map Strategists. The Road Map Strategist initiative is the first nationwide effort to build local health department capacity to address cognitive health and dementia. Eight local health departments were selected through a competitive application process for the cohort. This will supplement the ongoing technical assistance provided by CDC in guiding, developing, and integrating risk reduction into new BOLD state plans.
For more information, see:
- https://learn.astho.org/p/hbi-action-plan#tab-product_tab_overview
- https://www.cdc.gov/aging/healthybrain/Indian-Country-resources.html
- https://www.cdc.gov/aging/partnership/partner-resources/index.html
- https://astho.org/Healthy-Aging/
- https://astho.org/generickey/GenericKeyDetails.aspx?contentid=20481&folderid=5162&catid=0
CDC’s weekly newsletter, Alzheimer ’s Disease and Healthy Aging, disseminated information on brain health, risk reduction, caregiving, SCD, general health, emergencies, care planning, and COVID-19 guidance to over 67,000 subscribers, which includes many public health professionals. The newsletters are a primary channel for disseminating information about new articles, tools, resources, and webinars related to brain health to an active and engaged audience.
CDC is developing a scientific paper examining the association between identified risk factors and SCD using the BRFSS. The findings of this research will be used to establish programmatic priorities and to monitor progress at the state and national levels.
(ONGOING) Action 6.C.3: Educate the public health workforces on Alzheimer’s disease and related dementias risk factors
Lead Agency: CDC
CDC has developed a Public Health Curriculum, a comprehensive course addressing cognitive health, cognitive impairment, and dementia, for use by undergraduate faculty in schools and programs of public health and related disciplines. This curriculum is aligned with the Core Competencies for Public Health Professionals. The curriculum is available free of charge and consists of four modules designed to be used individually or as a whole, each with slides and a faculty guide. The curriculum is also relevant to other audiences for broader reach. The course was available updated in late 2019, with additional enhancements, including video modules, added in 2021.
For more information, see:
- https://www.cdc.gov/aging/services/index.htm
- http://www.phf.org/resourcestools/Pages/Core_Public_Health_Competencies.aspx
CDC is collaborating with the Dementia Risk Reduction PHCOE to translate existing and emerging science around modifiable risk factors for cognitive decline and dementia into actionable and targeted public health interventions, messaging, and campaigns; make these approaches highly accessible to the public health community and the general public; work with public health agencies and their partners to increase the use of these risk reduction strategies; and continuously update and improve the approaches through feedback, evaluation, and quality improvement.
For more information see:
(NEW) Action 6.C.4: Improve nutrition by lowering sodium in the food supply
Lead Agency: FDA
Excess sodium can raise blood pressure, which can increase the risk for multiple chronic conditions including AD/ADRD. Lowering blood pressure has been found to reduce the risk of developing cognitive impairment, a common precursor of AD/ADRD. According to the Dietary Guidelines for Americans, people living in the United States consume on average 3,400 milligrams (mg) of sodium per day -- nearly 50% more than the 2,300 mg limit recommended by for people 14 years and older. The majority of sodium consumed comes from processed, packaged, and prepared foods, which makes it difficult to monitor and limit sodium intake.
To address this, the FDA is taking an iterative approach that includes establishing voluntary sodium targets for industry, monitoring and evaluating progress, and engaging with stakeholders, in order to gradually reduce sodium across the food supply, including processed and restaurant foods. The FDA issued the final guidance with voluntary short-term targets for reducing sodium in commercially processed, packaged, and prepared food over the next two and a half years. The approach supports sodium reduction efforts already made by industry, provides common targets for defining and measuring progress, and provides companies with the flexibility and time to meet these targets. The FDA expects to issue revised subsequent targets in the next few years to facilitate a gradual, iterative process to reduce sodium intake.
For more information see:
(NEW) Action 6.C.5: Promote physical activity among older adults
Lead Agency: ODPHP
HHS released the Physical Activity Guidelines for Americans 2nd edition in 2018. The Physical Activity Guidelines for Americans is a flagship resource for health professionals and policy makers that provides recommendations on how everyone can improve their health through regular physical activity. The Guidelines describe the brain health benefits of physical activity, including reduced risk of AD/ADRD and improved cognition (executive function, attention, memory, crystallized intelligence, and processing speed).
The PAGAC Brain Health subcommittee examined the literature related to physical activity and cognition, identified key research recommendations and rationales for future exploration.
HHS intends to release a midcourse report in 2023 focused on strategies to increase physical activity among older adults.
The Office of Disease Prevention and Health Promotion (ODPHP) is also seeking input on how to help professionals, educators, and others working to promote or implement physical activity among older adults. In 2021, ODPHP released a Federal Register Notice seeking: (1) Nominations to serve on a subcommittee of the President’s Council on Sports, Fitness & Nutrition, which will be convened to conduct a literature review and summarize findings to support the development of the report; and (2) Written comments on how this report can best support decision makers, health professionals, educators, and others working to promote or implement physical activity among older adults.
For more information see:
- https://health.gov/our-work/nutrition-physical-activity/physical-activity-guidelines/current-guidelines
- https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
- https://health.gov/sites/default/files/2019-09/09_F-3_Brain_Health.pdf
- https://health.gov/
Strategy 6.D: Expand Interventions to Reduce Risk Factors, Manage Chronic Conditions, and Improve Well-Being through the Aging Network
The Aging Network is a national structure of state and local agencies that provide services to older adults with the aim of helping them remain in their homes and communities. Many older adults are already connected to Aging Network providers in their communities. These existing connections of trust provide an invaluable foundation for spreading awareness and implementing interventions on risk reductions to older adults, tribes, communities, states, and territories. By engaging with existing community organizations, risk reduction interventions can be tailored to fit the sociocultural needs of local communities.
(ONGOING) Action 6.D.1: Ensure older adults have access to nutritious meals through home-delivered and congregate meal programs
Lead Agency: ACL
Through the OAA Nutrition Program, ACL’s Administration on Aging (AoA) provides grants to states to help support nutrition services (home-delivered and congregate meals) for older people throughout the country. Nutrition services provide an opportunity to link to other supportive in-home and community-based supports from which older people may benefit.
Designed to promote the general health and well-being of older individuals, the services address hunger, food insecurity and malnutrition of older adults; promote the health and well-being, promoting healthy nutrition behaviors. The onset of COVID-19 highlighted the increased nutritional needs of the nation’s older adults, bringing hidden hunger and the needs of under-served individuals into the spotlight.
(ONGOING) Action 6.D.2: Expand the delivery of health and wellness programs to older adults in every community
Lead Agency: ACL
Through their Capacity-Building and Sustainable Systems initiatives ACL continues to expand delivery of health and wellness programs in more communities across the Nation. The Capacity-Building grants support building capacity in areas with no or limited program infrastructure to introduce and deliver evidence-based health and wellness programs, as well as chronic disease self-management support programs within under-served geographic areas and/or populations. The Sustainable Systems grants focus on the development of integrated, sustainable systems for delivering evidence-based self-management support programs. Expanded availability of health and wellness programs in historically under-served communities, many of whom are at high risk of developing cognitive impairment.
(ONGOING) Action 6.D.3: Identify the most promising health promotion and disease prevention interventions for dissemination through the Aging Network
Lead Agency: ACL
ACL, through grants with the National Council on Aging and the Evidence-Based Leadership Council, supports the vetting and identification of promising health and wellness community-based evidenced-based programs to support older adults and people with disabilities remaining in their homes and communities. Interventions are assessed to determine whether they meet the OAA Title III-D criteria for evidence-based programs, thus making them eligible for funding with OAA Title III-D dollars.
(ONGOING) Action 6.D.4: Expand access to evidence-based health promotion and disease prevention programs
Lead Agency: ACL
The OAA, under Title III, makes funds available to support the delivery of evidence-based programs designed to improve health and well-being, and reduce disease and injury. Through Title III, the aging services network is able to advance wider implementation of disease prevention and health promotion evidence-based programs demonstrated to improve the health of older adults. ACL developed an evidence-based definition to assist states in developing their own Title III-D guidance through which a variety of interventions are implemented and older adults are learning to manage chronic conditions (diabetes, heart disease, arthritis, chronic pain, and depression) which are known to contribute to increased risk for cognitive impairment later in life.
Strategy 6.E: Address Inequities in Risk Factors for Alzheimer’s Disease and Related Dementias Among Marginalized Populations
Black, Hispanic, and low income populations face a higher risk of AD/ADRD. Structural inequities are an important cause of this difference, including but not limited to underinvestment in education systems, less walkable communities, decreased access to nutritious food, barriers to health care access and low quality of care in their communities. To reduce existing disparities in the incidence of AD/ADRD risk reduction, interventions should be tailored to meet the needs of each community with cultural competence and equity as the primary focuses. This requires that addressing SDOH, entrenched systemic racism, and other forms of discrimination be prioritized, rather than focusing solely on individual behaviors.
(ONGOING) Action 6.E.1: Support the development of programs and materials designed to increase awareness of the importance of brain health in culturally and linguistically appropriate ways
Lead Agency: CDC
CDC’s NBHCAA is raising awareness of the issues of cognitive health among Black Americans by working through networks of faith-based institutions, and by establishing partnerships with organizations and individuals dedicated to NBHCAA’s mission. CDC is working on a training program geared towards Black American health professionals to raise awareness and diagnostic proficiency regarding cognitive health. CDC is expanding on the foundational work of NBHCAA to further education of health professionals about risk reduction as part of the HBI.
CDC’s HBI is increasing tailored messaging related to cognitive impairment, COVID-19, brain health, and AD/ADRD to Black and Hispanic populations across the United States. In 2021, three digital education events -- #SaludTues Twitter Chats -- amplified educational content, including CDC resources, about cognitive impairment and AD/ADRD as public health issues among diverse audiences. These three events were held in partnership with more than ten community-partners, generating 19.7 million impressions. Relatedly, a virtual congressional briefing was held on July 20 titled Brain Health Equity and the Social Determinants of Health, Congressional Districts and Alzheimer’s Prevalence Among Communities of Color.
In 2020, CDC funded three organizations for 5 years to tailor brain health messaging for four populations disproportionately affected by dementia: persons with IDD and Hispanic, Black, and AI/AN individuals. In 2021, a virtual congressional briefing was held in July entitled Brain Health Equity and the Social Determinants of Health, Congressional Districts and Alzheimer’s Prevalence Among Communities of Color. There was also the virtual HealthMatters Webinar Series, which reached over 400 attendees, and authored two publications on persons with IDD and AD/ADRD.
For more information, see:
(ONGOING) Action 6.E.2: Support the development of programs and materials designed to increase awareness of the importance of brain health for Tribal communities in culturally sensitive ways
Lead Agencies: CDC, IHS
Partners: private organizations, ASTHO
Through CDC’s HBI, in 2021 IA2, which provides support to AI/AN adults, developed a new website, AIAN Brain Health, which features a robust online brain health resource library. IA2 has collaborated with the Dementia Friends Program to provide tribes, UIH organizations, and Alaska Native communities with training and content from this program. IA2 also gathers, creates and distributes information and resources developed by and for AI/AN communities to improve the public health response to AD/ADRD. These resources are continuously updated to their brain health resource library for Tribal and UIH organizations.
Multiple sessions and trainings on AD/ADRD were hosted at the American Indian Elders Conference hosted by NICOA.
CDC partnered with the ASTHO and the Alzheimer’s Association to develop culturally sensitive materials to educate and empower tribes and Tribal populations about brain health and caregiving. In 2021, the materials were adapted to be customizable templates with logos, websites and images that focus on cardiovascular risk factors related to brain health and caregiving issues for Tribal communities.
In conjunction with the Alzheimer’s Association and other partners, CDC is developing a special edition of the Public Health Road Map for Tribal Communities, the HBI Road Map for Indian Country. The original Road Map focuses on issues pertinent to state and local public health agencies and their partners. A companion Road Map for Indian Country has been designed specifically for public health systems serving AI/AN adults. Additionally, there are several companion materials to support brain health in Tribal communities developed by CDC in partnership with ASTHO and the Alzheimer’s Association. IA2, a recipient of the HBI support, and the Northwest Portland Area Indian Health Board are developing, tailoring, and disseminating AD/ADRD materials and resources to AI/AN communities. CDC and the Alzheimer’s Association, in collaboration with IHS, also produced infographics sharing data from the 2015-2018 BRFSS describing caregiving and SCD among AI/AN adults.
For more information, see:
- http://www.aianbrainhealth.org/
- https://www.dfamerica.org/
- https://iasquared.org/brain-health/resource-library/
- https://www.cdc.gov/aging/partnership/partner-resources/index.html
- https://www.cdc.gov/aging/healthybrain/Indian-Country-resources.html
- https://www.astho.org/healthy-aging/healthy-heart-healthy-brain/
(NEW) Action 6.E.3: Reduce financial barriers to hearing aids for individuals with hearing loss
Lead Agency: FDA
As described in further detail under Action 6.B.2 above, the FDA recently issued a Proposed Rule to establish a new regulatory category for OTC hearing aids and to make related amendments to update the regulatory framework for hearing aids. When implemented, this Proposed Rule will increase the availability of less costly, OTC options for hearing aids, and therefore promote broader and more equitable access to these devices.
Strategy 6.F: Engage the Public about Ways to Reduce Risks for Alzheimer’s Disease and Related Dementias
Greater public awareness about potential risk factors and steps to modify those risk factors may encourage individuals and families to make changes that preserve cognitive health and promote healthy aging overall and connect them to resources and services that can help. Dementia is one of people’s most feared health conditions, which may influence an individual’s views about risk reduction messages and their interest in interventions to reduce their individual burden of risk factors for AD/ADRD. Furthermore, sharing information on SDOH and system-level risk factors can focus and help coordinate facilitate positive community and infrastructure changes.
(NEW) Action 6.F.1: Target and coordinate public health campaigns aimed at reducing risk factors
Lead Agencies: ACL, NINDS/NIA/NIH, CDC
Several federal agencies have developed public messaging campaigns to raise awareness of actions that individuals and communities can take to improve brain health and potentially reduce the risk of dementia. Federal agencies will expand partnerships and coordinate messaging efforts across public and private entities. Recent efforts have also included a stronger emphasis on tailoring messages to at-risk individuals, such as Black and Hispanic individuals and women. New and ongoing public messaging efforts should continue to enhance the cultural competence and assess the effectiveness of messaging across different populations.
(ONGOING) Action 6.F.2: Provide information to the public on brain health
Lead Agencies: CDC, HRSA, NINDS/NIA/NIH, ACL
CDC disseminates a weekly newsletter, Alzheimer’s Disease and Healthy Aging, to more than 67,000 subscribers. It is a primary channel for disseminating information about new articles, tools, resources, and webinars related to brain health. CDC recently launched a series of podcasts titled Aging and Health Matters that includes short discussions on issues in older adult health, including AD/ADRD and caregiving. Topics include: Healthy Body, Healthy Brain, Alzheimer’s Disease-Genes do not equal Destiny, and Memory Problems? Talk to your Doctor among others. CDC has a second newsletter, Alzheimer’s Disease and Healthy Aging Tribal Newsletter, is sent regularly to more than 470 subscribers interested in issues for AI/AN Elders.
CDC partners with the ASTHO to produce a series of products to support public health agencies in identifying priorities, areas of synergy within existing or upcoming jurisdictional plans, and opportunities for integrating cognitive health into public health efforts as guided by the HBI Road Maps. Products designed to facilitate implementation of the HBI Road Maps include a series of HBI Action Institutes across the country in each HHS region, health communication materials for AI/AN communities, and a series of recorded webinars to promote the importance of public health in addressing brain health.
For more information, see:
- https://www.astho.org/Healthy-Aging/
- https://tools.cdc.gov/medialibrary/index.aspx#/podcastseries/id/302101
- https://tools.cdc.gov/medialibrary/index.aspx#/media/id/397843
- https://tools.cdc.gov/medialibrary/index.aspx#/media/id/402234
- https://tools.cdc.gov/medialibrary/index.aspx#/media/id/408892
Grant recipients of HRSA’s GWEP are expanding their social media footprint by developing Tweets, public service announcements, videos, and public radio and television spots. In 2021, HRSA grantees will incorporate approaches to identify and mitigate AD/ADRD risk factors into their training materials and disseminate information on risk reduction through social media channels.
The campaign, “What is Brain Health?” formerly managed by ACL was transferred to NIA in 2017 and retired in 2020. NIA maintains a web portal on Cognitive Health and Older Adults, which was updated in 2020. Also in 2020, NIA published 11 lay-friendly stories that highlight recent research results in brain and cognitive health.
For more information, see:
- https://www.nia.nih.gov/health/topics/brain-health
- https://www.nia.nih.gov/health/brain-health-resource
- https://www.nia.nih.gov/health/cognitive-health
In 2012 ACL, in partnership with NIH/NIA and CDC, created their Brain Health: You Can Make a Difference! curriculum/toolkit. The curriculum/toolkit was updated and simplified in 2018 and includes modules on brain health basics; medications and the brain; brain injury; and dementia, as well as complimentary evaluative tools to demonstrate training outcomes. Through ADPI, ACL’s grantees use these tools to boost the dementia-capable services and supports in their states and communities. The available tools provide information on the risk factors associated with developing dementia, knowledge of the signs of cognitive impairment, and management of symptoms of people living with dementia.
For more information, see:
- https://tools.cdc.gov/medialibrary/index.aspx#/podcastseries/id/302101
- https://tools.cdc.gov/medialibrary/index.aspx#/media/id/397843
- https://tools.cdc.gov/medialibrary/index.aspx#/media/id/402234
- https://tools.cdc.gov/medialibrary/index.aspx#/media/id/408892
- https://acl.gov/brain-health
In 2020, all of the 48 HRSA-funded GWEPs were funded to educate and train the public on brain health, including by sharing resources.
In 2021, the VA provided information about brain health on its Office of Geriatrics and Extended Care website. The information was developed by the VA GRECC program.
For information, see:
The State of Aging and Health in America: Data Brief Series, developed in collaboration with NACDD and CDC are topic-specific documents focusing on public health issues related to older adults. These briefs provide public health professionals with the most recent data available on health and aging-related conditions, including the importance of brain health, the management of chronic conditions, and caregiving burdens, to help identify needs and mitigate the future effects of a growing older population. The briefs also provide data by important breakdowns such as by state, age, gender, and ethnicity which can be useful for states and other stakeholders in making informed decisions and policies related to these issues.
For more information, see:
- https://www.cdc.gov/aging/data/index.htm
- https://www.cdc.gov/aging/healthybrain/Indian-Country-resources.html
- https://www.cdc.gov/aging/publications/briefs.htm
(NEW) Action 6.F.3: Enhance the reach and effectiveness of public health messaging on blood pressure control
Lead Agencies: NINDS/NIH, CDC
Researchers and public health officials have identified hypertension as one of the most modifiable risk factors for brain health and potentially dementia. CDC’s Million Hearts® as well as NIH’s “The Heart Truth” and “Mind Your Risks” all educate the public on the importance of reducing blood pressure among other modifiable risk factors. The “Mind Your Risks” campaign further stresses the link between uncontrolled blood pressure in midlife and increased risk for dementia. NIH is conducting qualitative research to refine and test the effectiveness of messaging approaches and campaign materials, including tailoring the campaign to better reach Black men in their early 20s-40s, who are at the highest risk for high blood pressure in midlife and dementia incidence in late-life.
For more information, see:
Appendix 1: List of Participating Departments and Agencies
Administration for Children and Families (ACF)
Administration for Community Living (ACL)
Administration on Aging (AoA)
Administration on Intellectual and Developmental Disabilities (AIDD)
Agency for Healthcare Research and Quality (AHRQ)
Centers for Disease Control and Prevention (CDC)
Centers for Medicare & Medicaid Services (CMS)
Consumer Finance Protection Bureau (CFPB)
Department of Defense (DoD)
Department of Health and Human Services (HHS)
Department of Housing and Urban Development (HUD)
Department of Veterans Affairs (VA)
Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
Food and Drug Administration (FDA)
Health Resources and Services Administration (HRSA)
Indian Health Service (IHS)
National Institute of Neurological Disorders and Stroke (NINDS)
National Institute on Aging (NIA)
National Institute on Minority Health and Health Disparities (NIMHD)
National Institutes of Health (NIH)
National Science Foundation (NSF)
Office of Global Affairs (OGA)
Office of Intergovernmental and External Affairs (IEA)
Office of the Assistant Secretary for Health (OASH)
Office of the Assistant Secretary for Public Affairs (ASPA)
Office of the Assistant Secretary for Planning and Evaluation (ASPE)
Office of the National Coordinator of Health Information Technology (ONC)
Office of the Surgeon General (OSG)
Office on Disability (OD)
Substance Abuse and Mental Health Services Administration (SAMHSA)
References
-
Hebert LE, Weuve J, Scherr PA, Evans DA. "Alzheimer’s Disease in the United States (2010-2050) Estimated Using the 2010 Census." Neurol, 2013; 80:1778-1783.
-
Burns A, Iliffe S. "Alzheimer's Disease." BMJ, 2009; 338:b158.
-
Alzheimer’s Disease Education and Referral (ADEAR) Center. Alzheimer’s Disease Fact Sheet. U.S. Department of Health and Human Services, National Institutes of Health. NIH Publication No. 11-6423. July 2011.
-
National Institute on Aging. 2009 Progress Report on Alzheimer’s Disease: Translating New Knowledge. U.S. Department of Health and Human Services, National Institutes of Health. NIH Publication No. 10-7500. November 2010.
-
Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology, 2015; 85(6):535-542. doi:10.1212/WNL.000000000000183.
-
Mahoney R, Regan C, Katona C, Livingston G. "Anxiety and Depression in Family Caregivers of People with Alzheimer’s Disease: The LASER-AD Study." J Am Geriatr Soc, 2005; 13(9):795-801.
-
Rajan KB, Weuve J, Barnes LL, et al. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020-2060). Alzheimer’s Dement. 2021; 1-10.
-
Rajan K, Weuve J, Barnes L, Wilson R, Evans D. “Prevalence and incidence of clinically diagnosed Alzheimer's disease dementia from 1994 to 2012 in a population study.” Alzheimer's & Dementia, 2019; 15(1):1-7. doi:10.1016/j.jalz.2018.07.216.
-
Samper-Ternent R, Kuo Y, Ray L, Ottenbacher K, Markides K, Al Snih S. (2012). Prevalence of Health Conditions and Predictors of Mortality in Oldest Old Mexican Americans and Non-Hispanic Whites. Journal of the American Medical Directors Association, 2-12; 13(3):254-259. doi:10.1016/j.jamda.2010.07.010.
-
Hurd M, Martorell P, Delavande A, Mullen K, Langa K. "Monetary Costs of Dementia in the United States." N Engl J Med, 2013; 368:1326-1334. http://www.nejm.org/toc/nejm/368/14/.
-
Maslow K. "How Many Hospital Patients Have Dementia?" In Silverstein N and Maslow K, eds. Improving Hospital Care for People with Dementia. New York, NY: Springer; 2006.
-
Magaziner J, et al. "The Prevalence of Dementia in a Statewide Sample of New Nursing Home Admissions Aged 65 and Older." Gerontol, 2000; 43(4):514-520.
-
Yaffe K, Fox P, Newcomer R, Sands L, Lindquist K, et al. "Patient and Caregiver Characteristics and Nursing Home Placement in Patients with Dementia." JAMA, 2002; 287:2090-2094.
-
Favreault, M. “Long-Term Services and Supports for Older Americans: Risks and Financing Research Brief.” U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation. February 2016. https://aspe.hhs.gov/basic-report/long-term-services-and-supports-older….
-
Taylor DH, Ezell M, Kuchibhatia M, Ostbye T, Clipp EC. "Identifying the Trajectories of Depressive Symptoms for Women Caring for Their Husbands with Dementia." J Am Geriatr Soc, 2008; 56(2):322-327.
-
Think Cultural Health Resource Library, HHS. How-To Guide: Providing CLAS. https://thinkculturalhealth.hhs.gov/assets/pdfs/resource-library/provid….
-
Ahearn EP, Szymanski BR, Chen P, Sajatovic M, Katz IR, McCarthy JF. "Increased Risk of Dementia Among Veterans with Bipolar Disorder or Schizophrenia Receiving Care in the VA Health System." Psychiatric Services, 2020; 71(10):998-1004. doi.org/10.1176/appi.ps.201900325.
-
Kuring JK, Mathias JL, Ward L. "Risk of Dementia in Persons who have Previously Experienced Clinically-Significant Depression, Anxiety, or PTSD: A Systematic Review and Meta-Analysis.” Journal of Affective Disorders, 2020; 274:247-261. doi.org/10.1016/j.jad.2020.05.020.
-
Rehm J, Hasan OSM , Black SE, Shield KD, Schwarzinger M. "Alcohol Use and Dementia: A Systematic Scoping Review." Alzheimers Research Therapy, 11: 1. doi:10.1186/s13195-018-0453-0.
List of Acronyms Used
A4 Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease
AAIC Alzheimer’s Association International Conference
AAPI Asian Americans and Pacific Islanders
ABC-DS Alzheimer’s Biomarker Consortium - Down Syndrome
ABCD Addressing Challenging Behaviors in Dementia
ACL HHS Administration for Community Living
ACPM American College of Preventive Medicine
ACTC Alzheimer’s Clinical Trials Consortium
AD Alzheimer’s Disease
AD-PM Alzheimer’s Disease and Precision Medicine
AD/ADRD Alzheimer’s Disease and Related Dementias
ADC Alzheimer’s Disease Center
ADEAR Alzheimer’s Disease Education and Referral
ADI-SSS Alzheimer’s Disease Initiative - Specialized Supportive Services
ADL Activity of Daily Living
ADNI Alzheimer’s Disease Neuroimaging Initiative
ADORE Alzheimer’s and Dementia Outreach, Recruitment, and Engagement Resources
ADPI Alzheimer’s Disease Programs Initiative
ADRC Alzheimer’s Disease Research Center
ADRD Alzheimer’s Disease-Related Dementias
ADSP Alzheimer’s Disease Sequencing Project
AHRQ HHS Agency for Healthcare Research and Quality
AI/AN American Indian/Alaska Native
AIDD ACL Administration on Intellectual and Developmental Disabilities
AIM Abuse Intervention Model
AlzPED Alzheimer’s Disease Preclinical Efficacy Database
AMP®-AD Accelerating Medicines Partnership® Program for Alzheimer’s Disease
AMP®-PD Accelerating Medicines Partnership® Program for Parkinson’s Disease
AoA ACL Administration on Aging
APS Adult Protective Services
ASPE HHS Office of the Assistant Secretary for Planning and Evaluation
ASTHO Association of State and Territorial Health Officials
AtG Alternatives to Guardianship
AWV Annual Wellness Visit
BOLD Building Our Largest Dementia infrastructure for Alzheimer's act
BPSD Behavioral and Psychological Symptoms of Dementia
BRFSS Behavioral Risk Factor Surveillance System
CADHC Community Adult Day Health Care
CAP Collaboration for Alzheimer’s Prevention
CARD Center for Alzheimer’s Disease and Related Dementias
CARES Act Coronavirus Aid, Relief, and Economic Security Act
CBO Community-Based Organization
CCBHC Certified Community Behavioral Health Clinic
CDC HHS Centers for Disease Control and Prevention
CDMRP Congressionally Directed Medical Research Program
CLC Community Living Center
CMMI CMS Center for Medicare and Medicaid Innovation
CMPRP Civil Monetary Penalty Reinvestment Program
CMS HHS Centers for Medicare & Medicaid Services
COVID-19 Novel Coronavirus
CROMS Clinical Research Operations and Management System
CSP Cooperative Studies Program
DetectCID Consortium for Detecting Cognitive Impairment, Including Dementia
DIAN-TU Dominantly Inherited Alzhiemer Network Trial Unit
DNA Deoxyribonucleic Acid
DoD U.S. Department of Defense
DoJ U.S. Department of Justice
DREAM Drug Repurposing for Effective Alzheimer’s Medicines
DYNASIM Dynamic Simulation of Income Model
EBP Evidence-Based Practices
EES Employee Education System
EHR Electronic Health Record
EJI DoJ Elder Justice Initiative
eLTSS Electronic Long-Term Services and Supports
FDA HHS Food and Drug Administration
FFS Fee-For-Service
FIC Fogarty International Center
FOA Funding Opportunity Announcement
FTD Frontotemporal Dementia
FY Fiscal Year
GACA Geriatrics Academic Career Award
GDO Global Dementia Observatory
GEOHealth Global Environmental and Occupational Health
GRECC Geriatric Research, Education, and Clinical Centers)
GWEP Geriatrics Workforce Enhancement Program
HABLE Health and Aging Brain Among Latino Elders
HABLE-AT(N) HABLE-Amyloid, Tau, and Neurodegeneration
HBI Healthy Brain Initiative
HBPC Home-Based Primary Care
HCAP Harmonized Cognitive Assessment Protocol
HCBS Home and Community-Based Services
HHS U.S. Department of Health and Human Services
HIT Health Information Technology
HRS Health and Retirement Study
HRSA HHS Health Resources and Services Administration
IA2 International Association for Indigenous Aging
IADRP International Alzheimer’s Disease Research Portfolio
IDD Intellectual and Developmental Disability
IHS HHS Indian Health Service
IMPACT IMbedded Pragmatic Alzheimer’s Disease and AD-Related Dementias Clinical Trials
INCLUDE Investigation of Co-occurring Conditions across the Lifespan to Understand Down Syndrome
INFINITY INtensive versus Standard Ambulatory Blood Pressure Lowering to Prevent Functional DeclINe In The ElderlY
IPF Inpatient Psychiatric Facility
LBD Lewy Body Dementia
LGBT Lesbian, Gay, Bisexual, and Transgender
LMIC Low and Middle-Income Country
LOAD Late-Onset Alzheimer’s Disease
LTSS Long-Term Services and Supports
M2OVE-AD Molecular Mechanisms of the Vascular Etiology of Alzheimer's Disease
MCI Mild Cognitive Impairment
MDT Multidisciplinary Team
MFH Medical Foster Home
mg Milligrams
MODEL-AD Model Organism Development and Evaluation for Late-onset Alzheimer's Disease
MRI Magnetic Resonance Imaging
NACC National Alzheimer's Coordinating Center
NACCHO National Association of County and City Health Officials
NACDD National Association of Chronic Disease Directors
NADRC National Alzheimer’s and Dementia Resource Center
NAPA National Alzheimer’s Project Act
NASEM National Academies of Science, Engineering and Medicine
NBHCAA National Brain Health Center for African Americans
NCAPPS National Center on Advancing Person-Centered Practices and Systems
NCHS NIH National Center for Health Statistics
NCRAD National Centralized Repository for Alzheimer’s Disease and Related Dementias
NCUIH National Council of Urban Indian Health
NHANES National Health and Nutrition Examination Survey
NHATS National Health and Aging Trends Study
NIA NIH National Institute on Aging
NIAGADS National Institute on Aging Genetics of Alzheimer’s Disease Data Storage Site
NICHD NIH National Institute of Child Health and Human Development
NICOA National Indian Council on Aging
NIH HHS National Institutes of Health
NIHB National Indian Health Board
NIJ DoJ National Institute of Justice
NIMHD NIH National Institute on Minority Health and Disparities
NINDS NIH National Institute of Neurological Disorders and Stroke
NLRC National Legal Resource Center
NORC National Ombudsman Resource Center
NOSI Notices of Special Interest
NRC-SDM National Resource Center for Supported Decision-Making
NTG National Task Group on Intellectual Disabilities and Dementia Practices
OAA Older Americans Act
OASH HHS Office of the Assistant Secretary for Health
ODPHP HHS Office of Disease Prevention and Health Promotion
ONC HHS Office of the National Coordinator for Health Information Technology
OTC Over-The-Counter
PAC Post-Acute Care
PASC Post-Acute Sequelae of COVID-19
PBRN Practice-Based Research Network
PET Positron Emission Tomography
PHCOE Public Health Center of Excellence
PHE Public Health Emergency
PPE Personal Protective Equipment
PPS Prospective Payment Systems
PRARP Peer Reviewed Alzheimer’s Research Program
PREVENTABLE Pragmatic Evaluation of Events and Benefits of Lipid-lowering in Older Adults
PTSD Post-Traumatic Stress Disorder
PwIDD-HBI People with IDD Healthy Brain Initiative
Q&A Question and Answer
QIO Quality Improvement Organization
REACH Resources for Enhancing Alzheimer’s Caregivers Health
REACH-VA Resources for Enhancing Alzheimer’s Caregivers Health-VA
REGARDS Reasons for Geographic and Racial Differences in Stroke
RITT Rural Interdisciplinary Team Training
SAMHSA HHS Substance Abuse and Mental Health Services Administration
SARS-CoV-2 Severe Acute Respiratory Syndrome Coronavirus 2
SBIR Small Business Innovation Research
SCD Subjective Cognitive Decline
SDM Supported Decision Making
SDOH Social Determinants of Health
SES Socioeconomic Status
SMI Serious Mental Illness
SPRINT-MIND Systolic Blood Pressure Intervention Trial - Memory and Cognition in Decreased Hypertension
STTR Small Business Technology Transfer
SUD Substance Use Disorder
T-MSIS Transformed Medicaid Statistical Information System
TBI Traumatic Brain Injury
TIP Treatment Improvement Protocol
TREAT-AD TaRget Enablement to Accelerate Therapy development for Alzheimer’s Disease
UIC University of Illinois at Chicago
UIH Urban Indian Health
UsA2 UsAgainstAlzheimer’s
VA U.S. Department of Veterans Affairs
VCID Vascular Contributions to Cognitive Impairment and Dementia
VHA VA Veterans Health Administration
