ASPE ISSUE BRIEF
Expanding the Use of Generic Drugs
By: ASPE Staff
December 1, 2010
This Issue Brief is available on the Internet at:http://aspe.hhs.gov/sp/reports/2010/GenericDrugs/ib.shtml
Printer Friendly (PDF) Version (19 pages) [Free PDF reader  ]
]
Contents
- Introduction
- Trends in Prescription Drug Spending and Generic Drug Use
- Legislative Origin of Generic Drugs
- Pathways through which Generic Drugs Reduce Health Care Costs
- Generic Drug Pricing
- Estimates of Savings from Generic Drugs
- Barriers to Greater Savings from Generic Drug Use
- State Generic Substitution Laws
- Availability of Generics
- Legal Settlement Pay-for-Delay
- Speed of Generic Drug Application Approvals
- Availability of Biosimilar Biologic Drugs
- Consumer Perceptions of Generics
- Prescriber Behavior
- Conclusions
Appendix A: List of State Laws Governing Generic Substitution by Pharmacists
Introduction
Dramatic growth in the use of generic drugs has generated substantial savings for American consumers. To examine how the Department of Health and Human Services (HHS) can encourage the use of generic drugs, Secretary Sebelius asked the Assistant Secretary for Planning and Evaluation (ASPE) to examine barriers to, and opportunities for, expanding the use of generic drugs. In this Issue Brief we summarize the findings of ASPEs review of the existing literature on this topic. We begin by briefly reviewing trends in generic drug use, the legislative origin of generic drugs in the United States, and the pathways by which generic drugs can reduce healthcare costs. Next, we examine the literature on generic drug pricing and the associated healthcare savings. We group information on barriers to generic drug use in three broad areas: state laws on generic substitution; factors related to availability of generics; and consumer and prescriber perceptions and behavior. Overall, we found that current levels of generic drug use are fairly high. There is potential for increased savings from generic drug use both through increased availability of generic drugs and through increased substitution, particularly therapeutic substitution as discussed below.
[ Go to Contents ]
Trends in Prescription Drug Spending and Generic Drug Use
The rate of generic prescribing for all prescriptions reached almost 75 percent in 2009, up from 57 percent in 2004. Generic drugs cost much less than their branded counterpart, so the high rate of generic prescribing resulted in billions of dollars of savings for the U.S. health care system. In 2010 to 2014, a number of blockbusters are projected to go off patent representing more than $209 billion in annual drug sales. This trend is projected to result in a decrease in branded sales of $113 billion(1). Maintaining or improving the generic prescribing rate is an important tool in efforts to control health care costs.
Legislative Origin of Generic Drugs
Innovative branded drugs seeking Food and Drug Administration (FDA) approval are required to submit to FDA a new drug application that includes clinical trial data that establishes the safety and efficacy of the new drug. Manufacturers of innovative branded drugs expend considerable time and resources in research and development and the approval of new drugs. Some estimates indicate that bringing a new drug to market costs more than a billion dollars and takes 10-15 years(2). Generic drugs are therapeutically equivalent to a branded drug. Generic drugs are required to have the same active ingredient and the same strength, dosage form, and route of administration as the brand name (or reference) product. Most generic drugs do not need to contain the same inactive ingredients as the brand product. In addition, a generic drug must be bioequivalent to the brand drug, that is there must be no significant difference between the generic and brand product in the rate or the extent to which the active ingredient is delivered to the patient. There can be some variability between brand name and generic drugs, but FDA puts limits on how much variability is acceptable. Drugs approved by FDA as therapeutically equivalent can be substituted with the expectation that the substituted product will produce the same clinical effect and safety profile as the prescribed product.
The Drug Price Competition and Patent Term Restoration Act of 1984 (frequently referred to as the Hatch-Waxman Act) amended the Federal Food, Drug, and Cosmetic Act to create an abbreviated pathway for approval of new drugs that are therapeutically equivalent to a branded drug. In addition to the patents that protect new inventions, Hatch-Waxman granted periods of exclusivity to manufacturers that had new drugs approved by FDA. If the branded drug is still in the period of exclusivity or protected under patent, generic versions of the branded drug can not be brought to market. The generic manufacturer files an abbreviated new drug application (ANDA) with the FDA. Once the drug is approved by the FDA and the branded drug is no longer protected by patent or exclusivity, the generic can be brought to market. Because they can reverse-engineer an innovator drug, and need not repeat safety and effectiveness studies, generic manufacturers can bypass the time and costs to develop a new drug and bring a drug to market with a much smaller investment. The Hatch-Waxman Act also offered incentives for generic manufacturers to challenge the patents of innovator drugs by offering a 180-day period of exclusivity for the first generic applicant to challenge the validity of a patent. The Act also allows exemptions from patent infringement for pre-application activities by generic drug sponsors.
Most experts agree that the Hatch-Waxman Act greatly increased the availability of generic drugs in the U.S. market. Prior to the Act, 35 percent of top selling innovator drugs no longer under patent had generic equivalents. By the late 1990s, almost all had generic equivalents(3).
[ Go to Contents ]
Pathways through which Generic Drugs Reduce Health Care Costs
Generic drugs can reduce healthcare costs through multiple pathways. These include generic substitution of drugs, substitution of drugs in the same therapeutic class, and reduction in the average branded prices paid by consumers due to generic substitution. The largest cost savings come from generic substitution of drugs by substituting the less expensive generic drug for the therapeutically equivalent branded drug. FDA publishes a list of drug products that are therapeutically equivalent in its publication Approved Drug Products with Therapeutic Equivalence Evaluations; however, generic substitution is not regulated by FDA. It can be done by the prescriber or the pharmacist, according to state laws and regulations. Because the generic drug is therapeutically equivalent to the branded drug, this substitution is straightforward. Generic substitution rates, the rate at which generic drugs are dispensed in the U.S., are almost 90 percent when there is a generic equivalent available(4). However, restrictions on the ability of pharmacists to carry out generic substitution vary by state.
Additional savings can result from therapeutic substitution. Therapeutic substitution is switching to a generic from a branded drug in the same therapeutic class. For example, a prescriber may switch a patient from the branded lipid-lowering statin, Lipitor (which as yet has no generic equivalent) to simvastatin, the generic equivalent of the branded lipid-lowering statin, Zocor. This substitution has to be done by the prescriber; a pharmacist can not substitute between drugs that are not therapeutically equivalent. Formularies(5), prior approval(6), prescriber incentives(7), and step therapy(8) create incentives for prescribers to substitute less expensive generics for branded drugs with the same indication. FDA does not regulate therapeutic substitution. Historically, there has been little evidence of significant savings from this type of substitution, but there is recent evidence that it is becoming a more important source of savings. For example, after introduction of generic simvastatin (therapeutically equivalent to Zocor), a study of Medicaid drug expenditures found that prescriptions of Lipitor declined from 43 percent of total statin use before the introduction of generic simvastatin to 31 percent a year after the introduction of generic simvastatin(9).
Another possible pathway for savings is a reduction in average branded prices paid by consumers resulting from generic substitution. A study by Rizzo and Zeckhauser found that a higher share of generic prescriptions result in lower average brand drug prices. The theory is that consumers are more likely to substitute generics for higher cost branded drugs and conversely less likely to substitute generics for lower cost branded drugs. This selective substitution would then effectively lower the average cost of branded drugs by leading brand name manufacturers to choose lower initial prices. This study found that a 10 percent increase in the generic substitution rate is associated with a 15.6 percent decline in the average price paid for branded drugs(10).
[ Go to Contents ]
Generic Drug Pricing
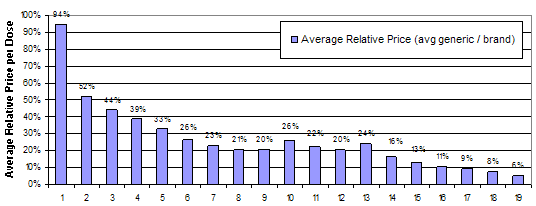
Generic drug manufacturers face much lower costs to enter the market than manufacturers of branded drugs. While estimates of the cost to bring a new branded drug to market are in excess of a billion dollars, the research and development costs for a new generic drug are only 1 to 2 million dollars(11). The relatively low costs to entry for generic drugs lead to increased competition, which drive prices for generic drugs down dramatically. Data from the National Association of Chain Drug Stores showed that the average retail prescription price for a generic drug in 2009 was $39.73, 76 percent less than $155.45, the average cost for a branded drug(12). The number of generic entrants appears to affect the price difference between branded and generic drugs. FDA analyzed the effect of generic drug entry on average prices for generics as a percentage of the price of the branded drug. They found that the first entrant has a relatively small effect on price, but subsequent entrants dramatically reduce the average relative price. Figure 1 below shows the average relative price for generics relative to branded drugs by number of generic entrants in the market(13). This analysis measures price as the price paid by the pharmacy, not the consumer. Pharmacies typically have higher markups for new generic drugs than branded drugs and older generic drugs(14). Also, the FDA analysis does not account for the fact that the most profitable markets attract the most generic competitors. As a result, the FDA analysis may overestimate the size of the price decrease for early generic entrants.
[ Go to Contents ]
Figure 1: Average relative price of generic to brand by number of generic competitorsGeneric Competition and Drug Prices
Number of Generic Manufacturers
Source: FDA analysis of retail sales data from IMS Health, IMS National Sales Perspective (TM), 1999-2004, extracted February 2005.
Estimates of Savings from Generic Drugs
In 2008, expenditures on prescription drugs reached $234 billion and were expected to grow 5.2 percent to $246.3 billion in 2009(15). The Congressional Budget Office (CBO) estimated savings from generic drugs for Medicare Part D in 2007. Total spending by the Part D program and its enrollees was $60 billion for one billion prescriptions in that year. Although 65 percent of prescriptions were filled by generic drugs, those prescriptions accounted for only 25 percent of total drug costs. CBO estimated that the availability of generics resulted in $33 billion in savings in 2007(16). A study by IMS Health, commissioned by the Generic Pharmaceutical Association, showed that savings from the use of generic drugs for the total healthcare system were estimated to be $139.6 billion in 2009(17).
A number of studies have estimated the potential savings from increasing the rate at which generics are dispensed. The CBO study found that increasing the generic substitution rate to 100 percent would result in an additional $900 million in savings(18) for the Part D program and enrollees. Haas et al analyzed data from the Medical Expenditure Panel Survey (MEPS) and estimated that if a generic had been substituted for the therapeutically equivalent branded drugs (increasing the generic substitution rate from 61 to 100 percent) that the total savings would have been $8.8 billion for the health care system in 2000. This estimate was likely an underestimate for 2000, as it did not include prescriptions for children and did not account for missing data in MEPS(19). Another study from Fisher and Avorn estimated spending by Medicaid for drugs for 48 states and the District of Columbia. In 2000, the total amount reimbursed by Medicaid in the studied states was $20.9 billion for drugs, of which $4.3 billion were for drugs that were available in generic forms. Fisher and Avorn found that an additional $229 million could have been saved if the generic substitution rate had been 100 percent. They found considerable state-level variation in potential savings ranging from 3.3 to 10.3 percent of total spending on drugs with generics available. However, the authors did not explore reasons for the variability between states. Another study of Medicaid spending by Alex Brill found that Medicaid could have saved $271 million of the total Medicaid spending of $21.8 billion by achieving a 100 percent generic substitution rate for 20 studied drugs. Across the 20 reference drugs the substitution rate ranged from 44 percent to 99 percent with an average generic substitution rate of 87 percent. This study found that most of the potential savings were concentrated in newly available generic substitutes due to a time lag in prescriber and pharmacist adoption of substitution with the newly available generic(20).
These studies consider the cost savings from achieving a 100 percent generic substitution rate. This is probably not a realistic or desirable goal. Generic drugs and their branded counterparts may differ in inactive ingredients, such as flavors, colors or binders and for some patients these differences can be important. For example, a patient with an allergy to a certain dye may be limited to using the branded drug or another generic that does not use that dye.
Studies of savings from Medicaid are uncertain due to rebates Medicaid receives from manufacturers. By law, Medicaid receives a larger rebate for branded than for generic drugs. These rebates are based on the average manufacturer price, which is proprietary, so researchers can not calculate the actual rebate. The difference in generic and branded drug price may be small in the first six months after introduction of the generic, when the first generic has exclusivity from other generic manufacturers. Therefore, in some cases generic drugs can be more expensive for Medicaid than branded drugs due to the larger rebate for branded drugs.
Further increases in savings are achievable from increasing therapeutic substitution. CBO examined seven therapeutic classes identified as having potential for therapeutic substitution and estimated that if all of the brand name drugs in those classes had been switched to a generic drug, prescription drug costs would have been reduced by $4 billion for the Part D program and its enrollees.
[ Go to Contents ]
Barriers to Greater Savings from Generic Drug Use
Barriers to the use of generic drugs can occur at a number of points, including state laws on generic substitution; factors related to availability of generics; and consumer and prescriber perceptions and behavior.
State Generic Substitution Laws
State laws regulate the practice of pharmacy. As a result, there is variation in requirements for when pharmacists can or must dispense generics among states. Some states require a pharmacist to substitute a therapeutically equivalent generic for a brand name drug, unless the physician specifies that a generic must not be substituted. Other states take a more permissive approach and allow, but do not require, pharmacists to substitute a generic drug, as long as the prescriber does not specify brand only. Some states impose an additional limitation that the pharmacist must get consent from the patient before substituting a generic. All states also allow the physician to specify that the brand name must be prescribed, although with different levels of effort from the physician. Appendix A provides state laws governing generic substitution by pharmacies.
A recent study of the effect of state generic substitution laws on drug spending under Medicaid found that state generic substitution laws can have a significant impact on drug spending. The study looked at spending by state on Zocor, generic simvastatin, and Lipitor in the first six quarters after the introduction of generic simvastatin. The study found a significant impact of patient consent laws on generic substitution. States that require patient consent had higher average prescription costs for Zocor and generic simvastatin combined than states that did not require patient consent. This difference was highest in the first quarter after patent expiration, $15.35, and declined to $2.68 by the fifth quarter after patent expiration. Similarly, six months after patent expiration, 98 percent of simvastatin prescriptions were written for generic simvastatin in states that did not require patient consent, while less than one third of prescriptions were filled by generic simvastatin in states that did require patient consent. The study did not find consistent differences in generic prescription rates between states that permitted pharmacists to prescribe generic alternative versus states that required pharmacists to prescribe a generic alternative(21). Pharmacists have a financial incentive to prescribe generics, as the mark up received by pharmacies is largest for new generics.
The study also looked at the impact of state laws and Medicaid policies, like prior authorization, on prescriptions for Lipitor, another statin in the same therapeutic class, but not therapeutically equivalent to simvastatin. Lipitor use declined from 43 percent of statin use before the introduction of generic simvastatin to 36 percent six quarters after the introduction of generic simvastatin. In states that required prior authorization for the prescription of Lipitor, Lipitor use was 31 percent lower than in states that did not. Other state generic substitution laws did not affect the levels of Lipitor use.
These findings for statin use in Medicaid may not be generalizable. The analysis of state laws for the introduction of a single generic drug, simvastatin, may differ from results for other drugs. Evidence from other studies suggests that savings vary by drug(22). More generics are likely to enter when the market for the branded drug is larger and more profitable. Also, drugs used by patients that are more responsive to price changes are also more likely to have generic competitors, such as drugs that are delivered in an inpatient setting or for chronic conditions(23).
There are also significant differences in the Medicaid and non-Medicaid populations. A survey on patients perceptions of generic medications found that patients that are older, patients that are poorer and patients with self-reported poor health were more likely to believe that brand name drugs are safer than generic drugs(24). Additionally, differences in co-pays between branded and generic drugs are much smaller in Medicaid than for most private insurance plans. Medicaid co-payments for retail drugs vary by state, but typically range from fifty cents to three dollars with lower amounts for generic and higher amounts for branded drugs(25). In the private insurance market, the average co-pay for a generic drug is $10, while the average co-pay for a preferred branded drug is $25 and for a nonpreferred branded drug the average co-pay is $43(26).
A few states also limit generic substitution by the pharmacist for drugs with a Narrow Therapeutic Index (NTI). Drugs with a narrow therapeutic index require careful titration and patient monitoring because there are relatively small differences between the effective dose and a toxic dose. NTI drugs include some anti-epileptic drugs, warfarin, and digoxin. FDAs policy regarding NTI drugs is that the generics are therapeutically equivalent to the branded drugs. However, some states require that generic versions can not be substituted for NTI drugs without the prescribers consent. No studies were identified that specifically address the impact of the state-level limitations on NTI drugs. Relatively few states impose this restriction and the impact of the state law would be difficult to disentangle from prescriber concerns about NTI substitution.
[ Go to Contents ]
Availability of Generics
The most important factor for whether consumers purchase generic drugs is the availability of a generic. Innovator drugs are protected from generic rivals by patents and by exclusivity.
Patents are issued by the U.S. Patent Office and offer 20 years of protection from competition. However, sponsors typically apply for a patent early in the drug development process and so many of the years of patent protection will be expended before the drug reaches the market. The Hatch-Waxman Act offers restoration of some of the years of patent protection expended during clinical testing and FDA review. Up to five years of patent term may be restored, with the total patent time after FDA approval limited to 14 years. The patent owner has to apply to the U.S. Patent Office for the restoration of patent life.
The Hatch-Waxman Act also provides innovator manufacturers with different periods of marketing exclusivity, depending upon the novelty of the drug. Marketing exclusivity is independent of patent protection. Some of the exclusivity periods delay the submission of an ANDA to the FDA for review, while others delay approval of an ANDA.
[ Go to Contents ]
Legal Settlement Pay-for-Delay
In some instances, a brand-name drug company may settle a patent challenge from a generic competitor by paying the generic company to delay entering the generic into the market. These settlements, called pay-for-delay or reverse payments, delay generic competition and the availability of generics. The FTC reports that there were 19 such agreements in fiscal year 2009, with each agreement on average delaying the availability of cost-saving generics by 17 months. The FTC also reported that, in January, 2010, such agreements were protecting at least $20 billion in sales of branded drugs from generic competition. The FTC estimated that pay-for-delay agreements cost American consumers $3.5 billion per year $35 billion over the next 10 years(27). The FTC has attempted to prosecute pay-to-delay agreements; however, these efforts have not been uniformly upheld in federal courts(28). The FTC has continued to litigate pay-for-delay cases in the courts and has recommended Congress pass legislation to prevent pay-for-delay agreements.
Speed of Generic Drug Application Approvals
As of June, 2010, FDA has 2,136 ANDAs pending, of these 850 are for generics not blocked by patents(29). This has resulted in a median approval time of 27 months for new generic drugs which includes time awaiting responses to information requests to sponsors. Because the average ratio of generic to branded drug price continues to decrease as additional generic drugs enter the market, delays for additional market entrants even beyond the first generic equivalent may reduce cost savings. However, delays in generic drug approval may not necessarily result in lost cost savings. First generics are rarely delayed by FDA review; most first generics are available when the patent expires. Often generic drug manufacturers submit applications to the FDA in advance of patent expiration or in anticipation of resolution of a patent dispute. These generic drugs, even if approved, will not be able to enter the market until the patent expires or is found invalid. ANDAs in the FDA backlog may also not be delayed by FDA review, as ANDAs pending at FDA could include ANDAs that have been returned to the sponsor with an information request. It is difficult to assess the economic significance of the pending applications not blocked by patents without analysis of the markets for the drugs that have pending applications. To speed generic approvals, FDA has requested authority to collect user fees for the review of generic drugs in the FY2011 Presidents Budget.
[ Go to Contents ]
Availability of Biosimilar Biologic Drugs
Another area of potential cost savings is the abbreviated pathway for approval of biosimilar biologic drugs under the Biologics Price Competition and Innovation Act of 2009 (BPCIA), within the Patient Protection and Affordable Care Act (PL 111-148). These biological drugs, regulated under the Public Health Service Act, are not eligible for the abbreviated approval pathway for generic drugs under the Hatch Waxman Act. The U.S. had $59 billion in sales in biologics in 2008(30). A recent estimate in December 2008 by the CBO suggests that the federal government, primarily Medicare, will save between $9 billion and $12 billion over 10 years by creating an abbreviated approval pathway for biosimilar biologic drugs(31). The FDA is currently working to implement the provisions of the BPCIA.
Consumer Perceptions of Generics
Although physicians and pharmacists act as patients agents in selecting appropriate drugs, patients have discretion in choosing whether to use generic drugs. Patients can communicate to their physicians or pharmacists their preference for branded drugs. Whether patients communicate a preference for branded drugs may depend on a number of factors, including drugs the patient is now using or has used in the past, knowledge about the specific generic or branded drugs, general knowledge about generics and branded drugs, and financial incentives to use generic drugs.
A recent survey of 2,500 commercially insured beneficiaries of a large, national pharmacy benefits manager found that although most consumers believe that generic drugs are a better value than branded drugs and are equally safe, this did not necessarily transfer into a preference for purchasing generic drugs. Fifty-six percent reported that Americans should use more generics, but only 36 percent of those surveyed preferred to take generics(32).
A study of the impact of alternative interventions on generic drug use examined claims level data from Blue Cross Blue Shield of Michigan(33). The authors examined a number of interventions: communication to plan members about generic drugs, statewide advertising, physician incentives, generic sampling, and doubling the co-pay for branded drugs. The study found that only the change in co-pay had an effect on the generic dispensing rate. However, the lack of impact of the physician incentives may be due to the sample of physician groups chosen for the study. The program targeted physicians in well-managed practices, which may have had little room to improve in the generic dispensing rates.
[ Go to Contents ]
Prescriber Behavior
Physician prescribing behavior is important to high generic prescription rates. Physicians may not prescribe generics due to habit or out of concerns about safety and efficacy of generic drugs. A study analyzing data from the National Ambulatory Medical Care Survey found that the majority of physicians referred to drugs by their brand name rather than generic name(34). This means physicians when prescribing may prescribe the branded drug out of habit rather than intention. In these cases, permitting pharmacists to substitute generic drugs can be an important factor in maintaining high generic substitution rates.
Differences between branded and generics exist that may compel the physician to prescribe the branded drug. The generic drug can differ from the branded in inactive ingredients, as long as this does not interfere with therapeutic equivalence. Patient sensitivities or allergies to inactive ingredients may necessitate using the branded version. Physicians may also believe that there are safety or efficacy differences between the branded and the generic. For example, some health care providers are unwilling to substitute NTI drugs. The American Academy of Neurologys official position is that The AAN opposes generic substitution of anticonvulsant drugs for the treatment of epilepsy without the attending physicians approval(35). In these cases the physician chooses deliberately to prescribe the branded drug. Physician education may have some influence on physician behavior, but this is likely to be difficult to change.
Unlike substitution of therapeutically equivalent generics for branded drugs, substitution of a generic drug for a branded drug that is not therapeutically equivalent, but has the same indication for a branded drug, requires that the physician make a decision to prescribe a generic. Physician education, incentives, and use of e-prescribing may influence physicians to change their behavior. E-prescribing is theorized to increase generic drug use by making information about available generics, formularies and cost information available to physicians at the time of prescribing. One study found that in the Blue Shield of California system that e-prescribing increased generic drug use by 5.9 percent(36).
[ Go to Contents ]
Conclusions
The rapid increase in generic prescribing makes estimates of savings and potential increases in savings from generic drug use a fast moving target. There is a clear consensus that generic savings are now a large and important source of health care savings. Increases in cost savings from greater substitution of generics for therapeutically equivalent drugs appear possible, though these increases are likely to be small relative to total spending on drugs. Limited evidence indicates that state prescribing laws that allow consumers more choice in whether to use generics reduce generic drug use.
Setting mechanisms to increase substitution of generic drugs for branded drugs that are not therapeutically equivalent, but have the same indication, has more potential for increasing cost savings. Increasing cost savings in this area relies most on educating physicians and setting mechanisms in place to encourage substitution. However, because in these cases the generics are not therapeutically equivalent, substitution must be done appropriately to ensure efficacy and patient safety.
Increased availability of generic drugs by eliminating pay-for-delay agreements and speeding ANDA reviews by FDA also shows promise for increasing savings. The FTC estimates that American consumers could save $35 billion over the next ten years due to earlier access to generic drugs if pay-for-delay agreements were eliminated. Although FDA ensures that reviews of ANDAs for first generics are not delayed, speeding reviews of subsequent generic competitors may further decrease generic prices, as research shows that more generic competitors lead to lower prices. However, without analysis of the pending ANDAs, the economic significance of the review delays can not be assessed.
The greatest and most certain potential for increased savings in the near future lies in increased availability of generic drugs through patent expiration for current blockbuster drugs. The high level of acceptance of generic drugs and mechanisms set in place to encourage generic substitution should result in continuing increases in savings from this avenue.
[ Go to Contents ]
Appendix A:
| State | Allows for Generic Substitution by Pharmacists if Brand Only Not Indicated by Physician | Mandates Generic Substitution by Pharmacists if Brand Only Not Indicated by Physician | Allows for Brand if Requested by Patient | Mandates Brand Only if Indicated by Physician | To Ensure Brand Name Only, Physician Must Indicate the Following on the Written Prescription OR Communicate Orally |
| Alabama | X | X | X | Sign the prescription signature line labeled May not Substitute or Dispense as Written. | |
| Alaska | X | X | X | In the physicians handwriting, the words Brand Medically Necessary must appear on the prescription. | |
| Arizona | X | X | X | Clearly display on the prescription DAW or other wording indicative of Substitution not Permitted. | |
| Arkansas | X | X | X | In the physicians handwriting, indicate that the product ordered should not be substituted. | |
| California | X | X | X | In the physicians handwriting, the words Do not substitute must appear on the prescription. | |
| Colorado | X | X | X | In the physicians handwriting, the words Dispense as Written must appear on the prescription. | |
| Connecticut | X | X | X | In the physicians handwriting, indicate that the product ordered should not be substituted. | |
| Delaware | X | X | X | Sign the prescription signature line labeled May not Substitute or Dispense as Written. | |
| Florida | X | X | X | In the physicians handwriting, the words Medically Necessary must appear on the prescription. | |
| Georgia | X | X | X | In the physicians handwriting, the words Brand Necessary must appear on the prescription. | |
| Hawaii | X | X | X | In the physicians handwriting, the words Brand Medically Necessary must appear on the prescription. Mandates Brand Only for Anticonvulsant Medications. | |
| Idaho | X | X | X | Physician must indicate Brand Only by checking the Brand Only box on the prescription. | |
| Illinois | X | X | X | Sign the prescription signature line labeled May not Substitute or Dispense as Written. | |
| Indiana | X | X | X | Sign the prescription signature line labeled May not Substitute or Dispense as Written. | |
| Iowa | X | X | X | Physician shall communicate to Pharmacist that product should not be substituted. | |
| Kansas | X | X | X | In the physicians handwriting, the words Dispense as Written must appear on the prescription. | |
| Kentucky | X | X | X | In the physicians handwriting, the words Do not substitute must appear on the prescription. | |
| Louisiana | X | X | X | Physician must indicate Brand Only by checking the Dispense as Written or DAW box on the prescription. | |
| Maine | X | X | In the physicians handwriting, the words Dispense as Written, DAW, Brand, or Brand Neccessary must appear on the prescription. | ||
| Maryland | X | X | X | Physician shall communicate to Pharmacist that product should not be substituted. | |
| Massachusetts | X | X | In the physicians handwriting, the words No substitution must appear on the prescription. | ||
| Michigan | X | X | X | In the physicians handwriting, the words Dispense as Written or DAW must appear on the prescription. | |
| Minnesota | X | X | X | In the physicians handwriting, the words Dispense as Written or DAW must appear on the prescription. | |
| Mississippi | X | X | X | Physician shall communicate to Pharmacist that product should not be substituted. | |
| Missouri | X | X | X | Sign the prescription signature line labeled May not Substitute or Dispense as Written. | |
| Montana | X | X | X | In the physicians handwriting, the words Brand Medically Necessary must appear on the prescription. | |
| Nebraska | X | X | X | In the physicians handwriting, the words Dispense as Written, DAW or similar statements must appear on the prescription. | |
| Nevada | X | X | X | In the physicians handwriting, the words Dispense as Written must appear on the prescription. | |
| New Hampshire | X | X | X | Physician must specify that the Brand is Medically Necessary. | |
| New Jersey | X | X | X | Physician must initial next to the option Do not Substitute on the prescription. | |
| New Mexico | X | X | X | In the physicians handwriting, the words No substitution or No sub must appear on the prescription. | |
| New York | X | X | In the physicians handwriting, DAW must appear on the prescription. | ||
| North Carolina | X | X | X | Sign the prescription signature line labeled May not Substitute or Dispense as Written. Narrow Therapeutic Range Drugs must be dispensed as originally prescribed. | |
| North Dakota | X | X | X | In the physicians handwriting, the words Brand Necessary must appear on the prescription. | |
| Ohio | X | X | X | In the physicians handwriting, the words Dispense as Written or DAW must appear on the prescription. | |
| Oklahoma | X | X | X | Physician shall communicate to Pharmacist that product should not be substituted. | |
| Oregon | X | X | X | In the physicians handwriting, the words No substitution or N.S must appear on the prescription. | |
| Pennsylvania | X | X | X | Physician shall communicate to Pharmacist that product should not be substituted. | |
| Rhode Island | X | X | X | In the physicians handwriting, the words Dispense as Brand Name Necessary must appear on the prescription. | |
| South Carolina | X | X | X | Sign the prescription signature line labeled May not Substitute or Dispense as Written. | |
| South Dakota | X | X | X | In the physicians handwriting, the words Brand Necessary must appear on the prescription. | |
| Tennessee | X | X | X | In the physicians handwriting, the words Dispense as Written, DAW, or other language of intent must appear on the prescription. | |
| Texas | X | X | X | In the physicians handwriting, the words Brand Necessary or Brand Medically Necessary must appear on the prescription. | |
| Utah | X | X | X | Sign the prescription signature line labeled May not Substitute or Dispense as Written OR in the physicians handwriting, the words Dispense as Written must appear on the prescription. | |
| Vermont | X | X | X | In the physicians handwriting, the words Brand Necessary or No substitution must appear on the prescription. | |
| Virginia | X | X | X | In the physicians handwriting, the words Brand Necessary must appear on the prescription. | |
| Washington | X | X | X | Sign the prescription signature line labeled May not Substitute or Dispense as Written. | |
| West Virginia | X | X | X | In the physicians handwriting, the words Brand Medically Necessary must appear on the prescription. | |
| Wisconsin | X | X | X | In the physicians handwriting, the words No substitutions or N.S must appear on the prescription. | |
| Wyoming | X | X | X | In the physicians handwriting, the words Brand Medically Necessary must appear on the prescription. | |
| From Epilepsy.com/Professionals. State Laws or Statutes Governing Generic Substitution by Pharmacists. 4/25/2007. http://professionals.epilepsy.com/page/statutes_by_pharmacists.html. | |||||
[ Go to Contents ]
Endnotes
1. Paul, SM, Mytelka, DS, Dunwiddie, CT, Persinger, CC, Munos, BH, Lindborg, SR, and Schacht, AL. How to improve R&D productivity: the pharmaceutical industrys grand challenge. Nature Reviews Drug Discovery. 2010;9:203-224.
2. DiMasi, JA and Grabowski, HG. The cost of biopharmaceutical R&D: is biotech different? Managerial and Decision Economics. 2007:28:469-479.
3. CBO. How Increased Generic Competition has Affected Drug Prices and Returns in the Pharmaceutical Industry. July 1998. Accessed on 9/30/2010 at http://www.cbo.gov/ftpdocs/6xx/doc655/pharm.pdf (PDF - 91 pages).
4. Shepard, Al. Generic Medicines: Essential contributors to the long-term health of society. IMS Health. 2010. Accessed at http://www.imshealth.com/imshealth/Global/Content/Document/Market_Measurement_TL/Generic_Medicines_GA.pdf (PDF - 16 pages).
5. Formularies are a list of drugs that a payer will pay for.
6. Requires the prescriber to obtain approval from the payer before prescribing certain drugs.
7. Providing incentives, usually monetary, based on physicians prescribing behavior.
8. Step therapy is when a less expensive drug is prescribed first and the patient is moved up to more expensive drugs if necessary.
9. Shrank, WH, Choudhry, NK, Agnew-Blais, J, Federman, AD, Libermand, JN, Liu, J, Kesselheim, AS, Brookhart, MA, and Fischer, MA. State generic substitution laws can lower drug outlays under Medicaid. Health Affairs. 2010; 29(7): 1383-1390.
10. Rizzo and Zeckhauser. Generic script share and the price of brand-name drugs: the role of consumer choice. Int J Health Care Finance Econ. (2009) 9:391-316.
11. H. Grabowski, Patents and New Product Development in the Pharmaceutical and Biotechnology Industries, Georgetown Public Policy Review 8, no. 2 (2003): 7-24.
12. From National Association of Chain Drug Stores. Facts at a Glance. Accessed on 9/30/2010 at http://www.nacds.org/wmspage.cfm?parm1=6536.
13. FDA. Generic Drug Prices. http://www.fda.gov/AboutFDA/CentersOffices/CDER/ucm129385.htm
14. Kina and Wosinka. Pharmaceutical Pricing in Handbook of Pricing Research in Marketing. Edward Alger Pub May 2009.
15. Andrea M. Sisko, Christopher J. Truffer, Sean P. Keehan, John A. Poisal, M. Kent Clemens, and Andrew J. Madison. National health spending projections: the estimated impact of reform through 2019. Health Affairs Web First, September 9, 2010.
16. CBO. Effects of using generic drugs on Medicares prescription drug spending. September, 2010.
17. GPhA. Savings achieved through the use of generic pharmaceuticals: 2000-2009.July, 2010.
18. Ibid.
19. Haas, JS, Phillips, K, Gerstenberger, SP, Seger, AC. Potential savings from substituting generic drugs for brand-name drugs: Medical Expenditure Panel Survey 1997-2000. Annals of Internal Medicine. 2005;142(11):891-897.
20. Brill, A. Overspending on multi-source drugs in Medicaid. AEI Health Policy Working Paper _2010-01
21. Shrank, WH, Choudhry, NK, Agnew-Blais, J, Federman, AD, Libermand, JN, Liu, J, Kesselheim, AS, Brookhart, MA, and Fischer, MA. State generic substitution laws can lower drug outlays under Medicaid. Health Affairs. 2010;29(7):1383-1390.
22. A Brill, A. Overspending on multi-source drugs in Medicaid. AEI Health Policy Working Paper _2010-01.
23. Scott Morton, F. (1997), The strategic response by pharmaceutical firms to the Medicaid most-favored customer rules, RAND Journal of Economics, 1997: 28(2):269-90.
24. Shrank, W, Cox, E, Fischer, MA, Mehta, J, Choudry, NK. Patients perceptions of generic medicines. Health Affairs, 2009;28(2):546-556.
25. Kaiser Family Foundation. Medicaid Benefits: Online Database, 2008. http://medicaidbenefits.kff.org/service.jsp?yr=4&so=0&cat=5&sv=32&gr=off&x=87&y=18
26. Pharmacy Benefit Management Institute. Prescription Drug Benefit Cost and Plan Design, Online Report, 2010-11, 2010. http://www.benefitdesignreport.com/CostSharingHighlights/RetailCopayments/tabid/84/Default.aspx.
27. Jon Leibowitz, Chairman, Fed. Trade Commission, Pay-for-Delay Settlements in the Pharmaceutical Industry: How Congress Can Stop Anticompetitive Conduct, Protect Consumers Wallets, and Help Pay for Health Care Reform (The $35 Billion Solution) at 8 ( June 23, 2009), available at: http://www.ftc.gov/speeches/leibowitz/090623payfordelayspeech.pdf (PDF -15 pages).
28. Pay-for-Delay: How Drug Company Pay-Offs Cost Consumers Billions, FTC Staff Study (Jan. 2010), http://www.ftc.gov/os/2010/01/100112payfordelayrpt.pdf (PDF - 16 pages).
29. FDA. FDA-TRACK CDER Office of Generic Drugs Dashboard. Accessed at: http://www.fda.gov/AboutFDA/Transparency/track/ucm206235.htm
30. IMS Biologics Webinar. 2009.
31. CBO. Budget Options Volume 1: Health Care. 2008
32. Shrank, W, Cox, E, Fischer, MA, Mehta, J, Choudry, NK. Patients perceptions of generic medicines. Health Affairs, March/April 2009;28(2):546-556.
33. OMalley, A, Frank, RG, Kaddis, A, Rothenberg, BM, and McNeil, BJ. Impact of Alternative Interventions on Changes in Generic Dispensing Rates. Health Services Research, 2006;41(5):1876-1894.
34. Steinman, MA, Chren, MM, Lendefeld, CS. Whats in a name? Use of brand-name versus generic drug names in United States outpatient practice. J Gen Intern Med. 2007;22(5):645-648.
35. Liow, K, Barkley, GL, Pollard, JR, Harden, CL, Bazil, CW. Position Statement on the coverage of anticonvulsant drugs for the treatment of epilepsy. Neurology. 2007;68:1249-1250.
36. Chang, C, Nguyen, N, Smith, A, and Huynh, D. Impact of electronic prescribing on outpatient prescription drug use and adherence in a network-model health plan. Presented at: Academy of Managed Care Pharmacy 22nd Annual Meeting and Showcase: April 9-10; San Diego.
[ Go to Contents ]